Atoms and Molecules
What is Atoms?
- It is the smallest particle of matter which take part in chemical reactions and does not lose its identity.
- Atoms are the basic units of matter and the defining structure of elements.
- An atom has no independent existence.
- Discovered by John Dalton
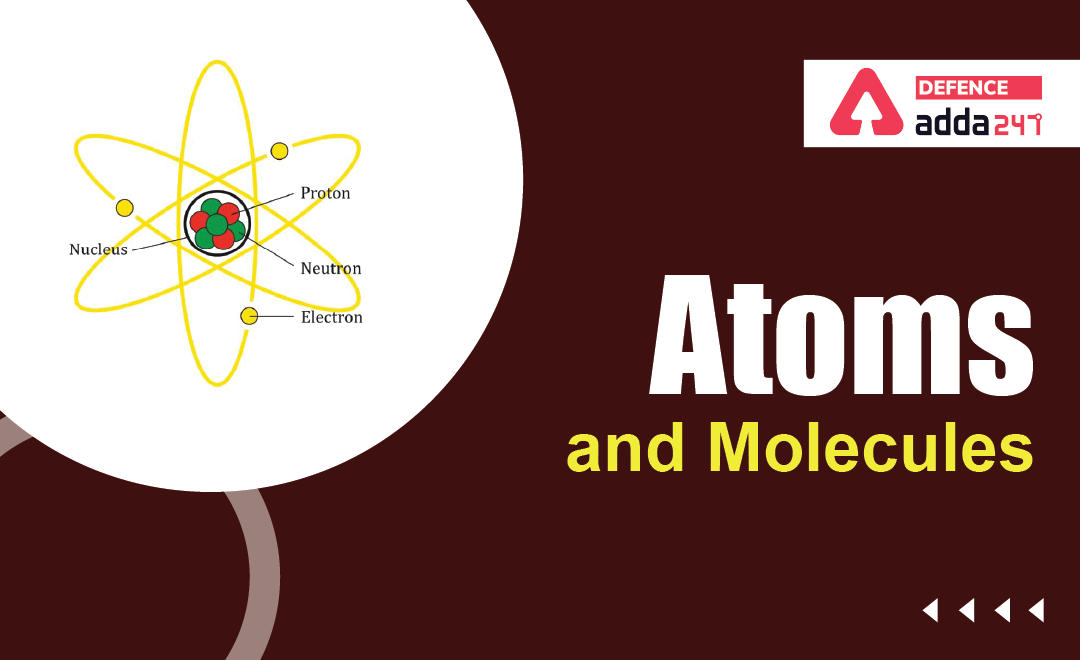
Structure of an Atom:
An Atom has two basic parts-
- Nucleus: Proton + Neutron
- Shell/Orbits: Electron
What is Molecules?
- A group of two or more than two atoms of the same or different elements that are chemically bonded together is called a molecule.
- A molecule can exist independently.
- g., H2, H2O, O2 etc.
Types of molecules:
- Homoatomic molecules
- Heteroatomic molecules
Homoatomic molecules-
- The molecules of same type of atoms.
- For example, a molecule of oxygen consists of two atoms of oxygen to form a diatomic molecule O2.
Heteroatomic molecules-
- The molecules of different type of atoms.
- For example, a molecule of carbon di oxide consists of one atom of carbon and two atoms of oxygen to form a triatomic molecule CO2.
Atomicity
- The number of atoms present in a molecule of an element or a compound is known as its atomicity.
Difference Between Atoms and Molecules
| S.N | Atoms | Molecule |
| 1 | Smallest particle in an Element | Two or more atoms combined together. |
| 2 | An atom is made up of neutrons, protons, and electrons. | A molecule of an element or a compound is made up of two or more atoms or the atoms of the constituent elements respectively. |
| 3 | Atoms of every element portray a certain degree of reactivity, except the noble elements. | Compared to atoms, molecules are less reactive. |
| 4 | Atoms have electrons on the outer shell. Due to this fact atoms may not remain stable all the time. | The formation of molecules take place to attain stability. |
| 5 | Example : O , H , P | Example : O2 , H2O , P4 , |
What is Atomic Mass
- Atomic mass in an atom or group of an atom is the sum of the masses of protons, neutrons and electrons.
- The electrons have very less mass in comparison with protons or neutrons so the mass of electrons is not influenced in the calculation.
Atomic mass = Mass of protons + Mass of neutrons + Mass of electrons

Average Atomic mass:
- The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance.
- Average atomic mass = f1M1 + f2M2 +… + fnMn where f is the fraction representing the natural abundance of the isotope and M is the mass number (Atomic mass) of the isotope.
- g., Chlorine consists of two major isotopes- 35Cl (75%) and 37Cl (25%).
- Hence Average atomic mass of Chlorine is 35.5
Molecular mass:
- It is the sum of atomic masses of all the constituent atoms of the molecule.
- g., molecular mass of CO2 is 44 amu/u.
Atoms and Molecules: Practice Questions
Q1. Which of the following defines the Mass number of an atom?
(a) number of protons + number of electrons
(b) number of neutrons + number of electrons
(c) number of protons + number of neutrons
(d) number of electrons
Ans-c
Q2. The number of electrons in one molecule of CO2 are –
- a) 22
- b) 44
- c) 66
- d) 88
Ans-a
Q.3- Atomic no. of argon?
(a)18
(b) 12
(c)15
(d) 11
Ans- a
Q4. The atomic number of an element is 17. The number of orbitals containing electron pairs in its valence shell is-
(a) Eight
(b) Six
(c) Three
(d) Two
Ans-c
Q5. If the atomic weight of an element is 23 times that of the lightest element and it has 11 protons, then it contains –
(a) 11 protons, 23 neutrons, 11 electrons
(b) 11 protons, 11 neutrons, 11 electrons
(c) 11 protons, 12 neutrons, 11 electrons
(d) 11 protons, 11 neutrons, 23 electrons
Ans- c
Q6. Molecular mass is defined as the:
(a) Mass of all the atoms, present in the molecule
(b) Mass of one atom compared with the mass of one atom of hydrogen
(c) Mass of one atom compared with the mass of one molecule
(d) None of the above
Ans (a)
Q7. The isotopes of an element have:
(a) Same number of neutrons
(b) Same atomic number
(c) Same mass number
(d) None of these
Ans-b
Q8. Constituents of atomic nucleus are-
(a) Electron and proton
(b) Electron and neutron
(c) Proton and neutron
(d) Proton, neutron and electron
Ans – c
Q9. The maximum number of electrons that can be accommodated in third shell ( n = 3) is:
(a)2
(b)8
(c)18
(d)10
Ans- c
Q10. Which of the following mostly accounts for the mass of an atom?
(a) neutrons
(b) Neutron and proton
(c) electron and proton
(d) electron and neutron
Ans-b




 Indian Navy SSR MR Recruitment 2025, App...
Indian Navy SSR MR Recruitment 2025, App...
 CDS 1 Exam Date 2025 Out, Check Exam Sch...
CDS 1 Exam Date 2025 Out, Check Exam Sch...
 AFCAT Exam Cut Off 2025 Out, Check Previ...
AFCAT Exam Cut Off 2025 Out, Check Previ...












