Acetic Acid Formula: Acetic acid is a carboxylic acid-containing chemical compound. The Acetic acid formula is CH₃COOH, which consists of a methyl group connected to a carboxyl functional group. The salts of Acetic acid and acetates are frequent components of animal and plant tissues and are generated during food digestion. In this article, we will learn more about Acetic Acid, its formula, preparations, and a wide range of applications.
What is Acetic Acid?
Acetic acid is an organic compound that is the second most basic carboxylic acid after formic acid. It is a carboxylic acid having a carboxyl functional group connected to a methyl group. Acetate is an acetic acid salt, ester, or acylal generated by the fermentation and oxidation of naturally occurring carbohydrates. It is an essential chemical reagent and industrial chemical used in the manufacturing of cellulose acetate, vinyl acetate, plastics, photographic films, textiles, and volatile organic esters, among other things.
Acetic Acid IUPAC Name
The Systematic Acetic acid iupac name is ethanoic acid, and its chemical formula is C2H4O2. The Latin word “acetum,” which is related to the word “acid,” is where the name “acetic acid” originates. Acetic acid has several other names, including ethanoic acid, ethylic acid, vinegar acid, and methane carboxylic acid. Vinegar is one of the most used common names for acetic acid. Although vinegar contains approximately 4-6% acetic acid in water. Glacial acetic acid refers to an undiluted solution of acetic acid.
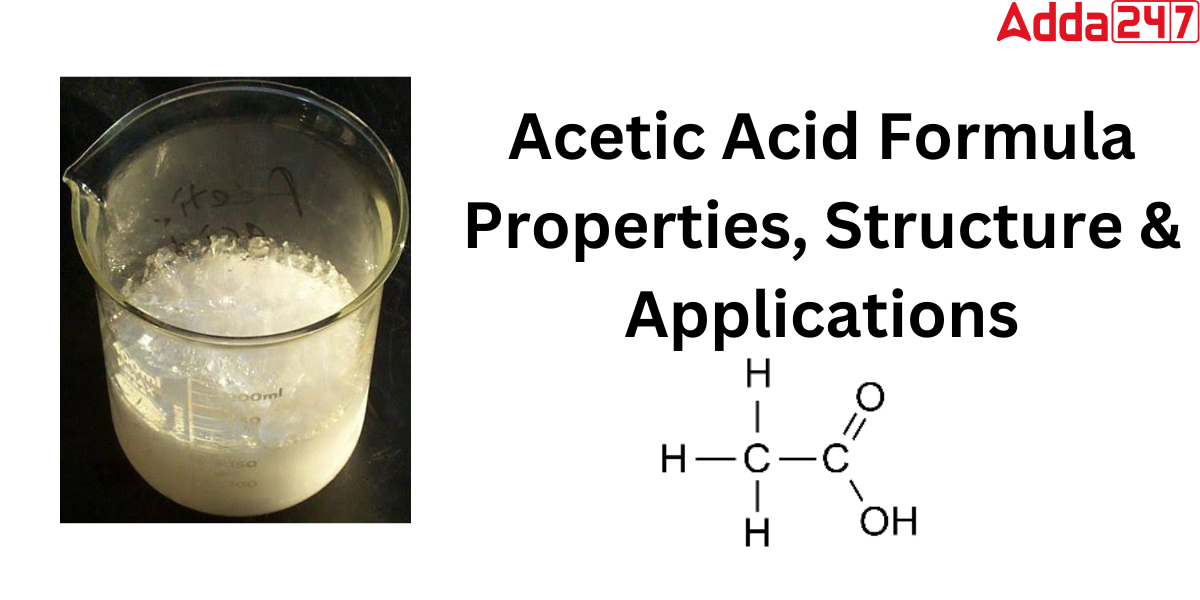
Acetic acid Chemical Formula
Acetic acid, commonly known as ethanoic acid, is a colorless, acidic liquid. The Acetic acid chemical formula is CH3COOH (sometimes written as CH3CO2H, C2H4O2, or HC2H3O2).
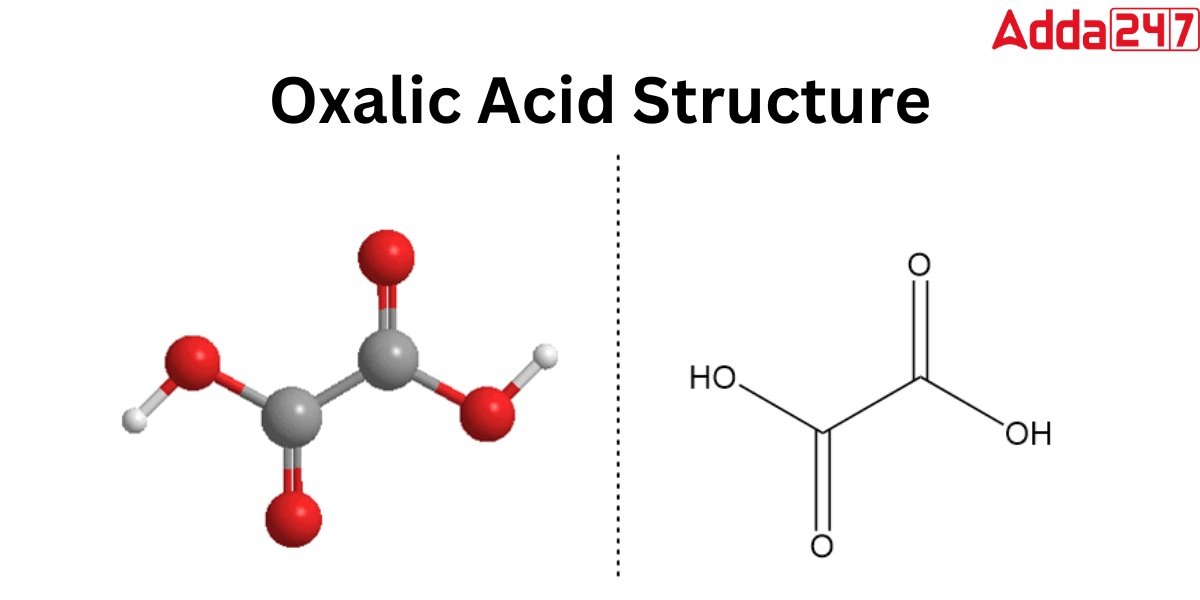
Acetic Acid Formula Structure
In organic Chemistry, Acetic acid is classified into the carboxylic acid family. The formula for acetic acid is CH3COOH, where a methyl group is connected to a carboxyl functional group. In the solid state of acetic acid, a chain of molecules may be seen, with individual molecules bonded to one another by hydrogen bonds. The structure of Acetic acid is defined as CH3(C=O)OH or CH3CO2H.
Acetic Acid Molor Mass
According to the Acetic Acid formula, it is made up of a methyl group linked to a carboxyl functional group. The molar mass of acetic acid is calculated to be 60.05 g/mol. It can be calculated as follows: (2 × 12.011) + (4 × 1.00794) + (2×15.999) g/mol= 60.05 g/mol
Properties of Acetic Acid
Acetic acid is an organic substance with the chemical formula acetic acid. CH3COOH After formic acid, acetic acid is the second most basic carboxylic acid. Examine the general properties of acetic acid.
- At STP, the melting and boiling temperatures of ethanolic acid are 289K and 391K, respectively.
- Acetic acid tastes sour and has a strong vinegar smell.
- Acetic acid has a liquid density of 1.049 g/cm-3 and a molar mass of 60.052 g/mol.
The carboxyl functional group of ethanolic acid has the ability to ionize the molecule, as demonstrated by the following reaction: Ethanoic acid + CH3COOH + CH3COO – + H+ - The proton release indicated in the equilibrium mechanism above is the fundamental reason acetic acid is acidic.
- In a water solution, ethanolic acid has an acid dissociation constant (pKa) of 4.76.
- Due to its favorable solvent properties and ability to mix miscibly with both polar and nonpolar compounds, acetic acid is a highly significant industrial solvent. Dimethyl terephthalate (DMT) is produced using it in large quantities.
Preparation of Acetic Acid
There are various methods to produce acetic acid. Methanol carbonylation is used in the industrial production of acetic acid. In this Reaction, A methyl iodide intermediate is created in this reaction between methanol and hydrogen iodide. After reacting this intermediate with carbon monoxide and treating the resulting chemical with water, the acetic acid product is generated. The chemical equations are associated with this procedure.
- CH3OH (methanol) + HI (hydrogen iodide) → CH3I (methyl iodide intermediate) + H2O
- CH3I + CO (carbon monoxide) → CH3COI (acetyl iodide)
- CH3COI + H2O → CH3COOH (acetic acid) + HI
Apart from the above-mentioned process for producing acetic acid, oxidizing ethylene (C2H4) results in acetic acid using a palladium catalyst and a heteropolyacid, as stated by the chemical reaction below.
O2 + C2H4 → CH3COOH
Chemical Reactions of Acetic Acid
Check out some Important Chemical Reactions of Acetic acid mentioned below:
- Acetic acid interacts with alkalis to produce acetate salts, as shown below.
CH3COOH + KOH → CH3COOK + H2O
- At temperatures above 440 C, this molecule breaks down, releasing either water and ethenone or methane and carbon dioxide.
CH3COOH + Heat → CO2 + CH4
Heat + CH3COOH → H2C=C=O + H2O
- By interacting with carbonates (along with carbon dioxide and water), acetic acid creates acetate salts. such responses comprise:
2CH3COOH + Na2CO3 (sodium carbonate) → 2CH3COONa + CO2 + H2O
CH3COOH + NaHCO3 (sodium bicarbonate) → CH3COONa + CO2 + H2O
- Several metals, including magnesium, zinc, and iron, corrode when exposed to acetic acid. These reactions result in the formation of acetate salts.
Mg (CH3COO)2 (magnesium acetate) + H2 = 2CH3COOH + Mg
Uses of Acetic Acid
Acetic acid has a variety of applications in human daily life, as well as in industries and laboratories. This is a list of some of the most important applications of acetic acid.
- Acetic acid is commonly used as vinegar in houses for a variety of chores such as cleaning, laundry, cooking, and many others.
- It’s also utilized in a number of textile printing methods.
- Its commercial applications include the production of vitamins, antibiotics, hormones, and organic compounds, as well as its use as a food ingredient (acidulant).
- Acetic acid is used as an antiseptic because of its antibacterial qualities.
- Acetic acid is used in the manufacturing of rayon fabric, rubber, and a variety of scents.
- Acetic acid has been used to treat cancer by injecting it directly into the tumor.
- Acetic acid is most commonly used in the food sector in industrial pickling procedures and condiments such as mayonnaise, mustard, and ketchup.
- Acetic anhydride, cellulose acetate, vinyl acetate monomer, acetic esters, chloroacetic acid, plastics, dyes, pesticides, photographic chemicals, and other products are made from acetic acid.
Acetic Acid as a Solvent
Acetic acid (CH3COOH), also known as ethanoic acid, is a weak organic acid with a pungent smell and a sour taste. It is a clear, colorless liquid and is an essential component in vinegar. While acetic acid is primarily known as an acid used for culinary and industrial purposes, it can also act as a solvent under certain conditions.
As a solvent, acetic acid has some unique properties and applications:
- Polarity: Acetic acid is a polar solvent, meaning it has a partial positive and partial negative charge within its molecule. This polarity allows it to dissolve a wide range of both polar and non-polar substances.
- Solubility: Acetic acid is miscible (able to mix) with water in all proportions, which makes it useful for creating aqueous solutions. It can also dissolve many organic compounds, salts, and even some inorganic materials.
- Applications:
- Laboratory Use: Acetic acid is often used in laboratories as a solvent for various purposes, including sample preparation, extraction, and chromatography. Its ability to dissolve a wide range of compounds makes it valuable in chemical research.
- Synthetic Chemistry: Acetic acid is used in certain chemical reactions as a solvent or as a reaction medium. For example, it’s used in the production of acetic anhydride, a chemical reagent used in the synthesis of other compounds.
- Textile Industry: Acetic acid is utilized in the textile industry for dyeing and finishing processes, where it helps dissolve dyes and facilitate dye uptake by fibers.
- Manufacturing: It’s also used in the production of various chemicals, plastics, and synthetic fibers.
- Cleaning and Degreasing: Acetic acid can be used as a cleaning agent to remove mineral deposits, scale, and stains due to its ability to dissolve mineral-based substances.
- Safety Considerations: While acetic acid can be used as a solvent, it’s important to note that it is an acid and can cause irritation and burns if it comes into contact with skin or eyes. Proper safety precautions, such as using gloves and protective eyewear, should be taken when handling concentrated acetic acid.
- Environmental Impact: Acetic acid is biodegradable and less harmful to the environment compared to some other organic solvents. This makes it a preferable choice in certain applications where environmental impact is a concern.
Overall, while acetic acid might not be as commonly used as other solvents like water, ethanol, or acetone, it does have its own niche applications in various industries and laboratory settings due to its unique properties.
Related Posts:








 CBSE Admit Card 2026 for Private & R...
CBSE Admit Card 2026 for Private & R...
 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...














