Acetone, a colorless and volatile organic compound, is one of the simplest and most commonly used ketones. Known for its distinct smell, acetone is widely utilized as a solvent in industries such as cosmetics, pharmaceuticals, and manufacturing. Its role as a primary component in nail polish remover highlights its ability to dissolve organic materials. Acetone is naturally present in the human body in small amounts as a byproduct of metabolism. In industrial applications, it plays a critical role in synthesizing plastics, fibers, and chemicals. This versatile chemical is both a practical tool and a subject of ongoing scientific study for sustainable applications.
Acetone
Acetone, also known as propanone or dimethyl ketone, is a ketone with the formula (CH3)2CO. It’s the simplest and tiniest in the ketone family. Acetone’s a colourless, extremely volatile, combustible liquid with a distinctive pungent odour. Acetone is miscible with water and is used as an organic solvent in business, at home, and in the laboratory. In 2010, around 6.7 million tonnes were manufactured globally, mostly for use as a solvent and the synthesis of methyl methacrylate (and thus PMMA) and bisphenol A. In organic chemistry, it is a common building block. Acetone is commonly used in home products such as nail polish remover and paint thinner. In the United States, it is free from volatile organic compound (VOC) regulations. Acetone is created and eliminated by regular metabolic processes in the human body. Acetone usually seen in the blood and urine. It is produced in greater quantities in diabetic ketoacidosis patients. Acetone has a modest possibility for causing reproductive difficulties, according to reproductive toxicity tests. Ketogenic diets, which raise blood levels of ketone bodies (acetone, -hydroxybutyric acid, and acetoacetic acid), are used to prevent epileptic seizures in infants and children with refractory epilepsy.
Acetone IUPAC Name
The IUPAC name for acetone is “propan-2-one.”
To break Acetone name down:
- “Propan” indicates that it is a three-carbon molecule.
- “-2-one” indicates that the functional group, a ketone, is located on the second carbon atom.
Putting it together, the IUPAC name for acetone is “propan-2-one.”
Acetone is a colorless, volatile liquid with a distinct sweet odor. Acetone is a simple organic compound with the chemical formula CH3COCH3. Acetone is a common solvent that is miscible with water, making it useful in various industrial and household applications. Here are some key characteristics and uses of acetone:
- Solvent: Acetone is a highly effective solvent for many substances, including oils, greases, resins, and certain plastics. It is commonly used to dissolve and remove paint, varnishes, and adhesives. Acetone’s ability to dissolve a wide range of compounds makes it valuable in many industrial processes.
- Nail polish remover: Acetone is a common ingredient in nail polish removers. It helps to break down and remove nail polish from the nails. However, due to its drying effect, it is recommended to use acetone-based nail polish removers sparingly and moisturize the nails afterward.
- Industrial applications: Acetone finds applications in various industrial processes. It is used as a solvent in the production of plastics, fibers, pharmaceuticals, and chemicals. It is also utilized in the manufacturing of coatings, adhesives, and cleaning agents.
- Laboratory use: Acetone is frequently used in laboratories as a solvent for chemical reactions, particularly for substances that are not soluble in water. It is also utilized as a cleaning agent for laboratory equipment due to its ability to remove residue and contaminants.
- Medical applications: In medicine, acetone can be used as a solvent for certain medications and as a component in some topical treatments. However, it is essential to note that acetone should not be used directly on the skin as it can cause drying and irritation.
- Household uses: Acetone is occasionally used in household applications, such as removing stains, cleaning surfaces, and degreasing metal components. However, it is important to exercise caution and use acetone in well-ventilated areas due to its flammable nature.
Acetone Formula
- Acetone or dimethyl ketone, is an organic compound having the formula as: (CH₃)₂CO.
- Acetone or dimethyl ketone is also known as propanone.
How to get Acetone Formula
The formula for acetone is derived from its structure and chemical properties as a simple ketone. It is C₃H₆O, indicating that it contains three carbon atoms, six hydrogen atoms, and one oxygen atom.
Steps to Understand the Formula:
- Functional Group Identification: Acetone belongs to the ketone family, characterized by a carbonyl group (C=O) bonded to two alkyl groups.
- Structural Composition: The central carbon atom of the carbonyl group is bonded to two methyl groups (CH₃), forming its simplest ketone structure.
- Chemical Notation: Combining all atoms gives the molecular formula C₃H₆O.
Acetone Structure
- The structure of Acetone or Propanone is:
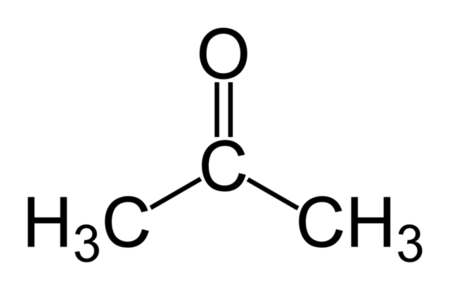
IUPAC Name of Acetone
- The IUPAC name of Acetone or dimethyl ketone is: propan-2-one
Acetone Molecular Weight
The molecular weight of acetone (chemical formula C₃H₆O) is approximately 58.08 g/mol. It is calculated by summing the atomic weights of its constituent elements:
-
- Carbon (C): 12.01 × 3 = 36.03
- Hydrogen (H): 1.008 × 6 = 6.048
- Oxygen (O): 16.00 × 1 = 16.00
Total: 58.08 g/mol.
Acetone Properties
- Acetone has been widely examined and is thought to have just a minor toxicity in regular use. If basic safeguards are taken, there is no compelling evidence of long-term health impacts.When consumed and/or inhaled, it is usually thought to have low acute and chronic toxicity. [69] Acetone is not currently considered a carcinogen, mutagenic chemical, or a source of long-term neurotoxicity.
- Acetone produces acetone peroxide as a byproduct when it is oxidised, which is a very unstable, main high explosive chemical. It can arise by accident, for example, when waste hydrogen peroxide is injected into acetone-containing waste solvent. Despite its straightforward chemical production, it is rarely employed due to its volatility.
- Acetone, like most ketones, exhibits the keto–enol tautomerism, in which the enol isomer (CH3)C(OH)=(CH2) is in equilibrium with the nominal keto structure (CH3)2C=O (prop-1-en-2-ol). Only 2.4×10−7% of the molecules in acetone vapour at room temperature are in the enol state. However, in some chemical processes, the enol form is crucial.
- Acetone is known to generate two types of polymers and (perhaps cyclic) oligomers. Units could be acetone molecules linked by ether bridges –O– produced from the opening of the double bond to form a polyketal-like (PKA) chain [–O–C(CH3)2–]n in one kind.
- Two acetone molecules react in the presence of adequate catalysts to generate diacetone alcohol (CH3)C=O(CH2)C(OH)(CH3)2, which dehydrates to mesityl oxide (CH3)C=O(CH)=C(CH3)2. This product can be combined with another acetone molecule, resulting in phorone and other chemicals with the loss of another molecule of water.
Acetone Reaction in Chemistry
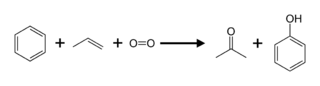
Acetone from Method 1
Propylene can be used to make acetone either directly or indirectly. The cumene process produces about 83 percent of acetone; as a result, acetone manufacturing is linked to phenol production. Cumene is produced by alkylating benzene with propylene, which is then oxidised by air to create phenol and acetone:
Other methods include directly oxidising propylene (Wacker-Hoechst process) or hydrating propylene to produce 2-propanol, which is then oxidised (dehydrogenated) to acetone.
Acetone from Method 2
Acetone was previously made by dry distilling acetates, such as calcium acetate in ketonic decarboxylation.
| Ca(CH3COO)2 → CaO(s) + CO2(g) + (CH3)2CO (v) |
Following that, in the manufacturing of Cordite, acetone was created utilising acetone-butanol-ethanol fermentation using Clostridium acetobutylicum bacteria, which was developed by Chaim Weizmann (later the first president of Israel) to aid the British war effort. When newer technologies with higher yields were discovered, the acetone-butanol-ethanol fermentation was eventually abandoned.
Related Post:








 NCHMCT JEE 2026: Exam Date, Online Regis...
NCHMCT JEE 2026: Exam Date, Online Regis...
 CUET PG 2026 Registration Started at exa...
CUET PG 2026 Registration Started at exa...
 CLAT Result 2026 Release Date, How to Do...
CLAT Result 2026 Release Date, How to Do...














