Table of Contents
Aluminium Fluoride Formula: Aluminium fluoride is a colourless chemical compound composed of aluminium and fluoride ions. The chemical formula of Aluminum fluoride is AlF3. Aluminium fluoride is a chemical compound composed of aluminium and fluorine atoms. It is mostly utilised in the manufacture of aluminium. The majority of aluminium fluoride is created by heating hydrogen fluoride with alumina or aluminium oxide to 700 degrees Celsius. Learn more about the aluminium fluoride formula, its chemical structure, properties, and applications in this article.
What is Aluminium Fluoride?
Aluminium fluoride is an inorganic compound with the formula AlF3 that has a wide range of applications in numerous industries and chemistry research. Aluminium fluoride is most usually found as a hydrate, the chemical formula for the most common of these hydrates is AlF3.5H2O, which means that there are seven molecules of water for there are seven molecules of water for every two molecules of aluminium fluoride in a crystal. It occurs naturally as oskarssonite and rosenbergite.
Aluminium Fluoride Formula
The name “aluminium fluoride” refers to its composition, which consists of aluminium and fluorine. The aluminium fluoride formula is AlF3 which shows that the chemical substance’s crystal lattice contains one aluminium ion (Al³⁺) and three fluoride ions (F), which maintain electrical neutrality. Each aluminium ion is surrounded by six fluoride ions, while each fluoride ion is surrounded by four aluminium ions.
Aluminium Fluoride Formula is AlF3
Structure of Aluminium Fluoride
In the Aluminium Fluoride Formula, the core atom is aluminium, which is surrounded by three fluorine atoms.The Lewis structure of aluminium fluoride is depicted in the figure below.
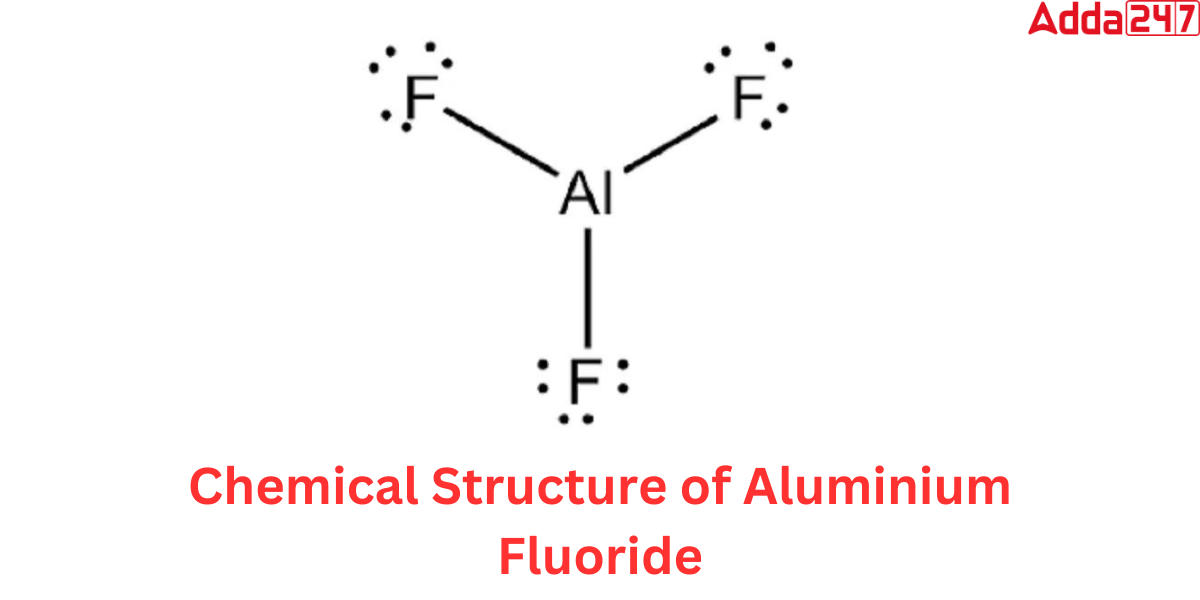
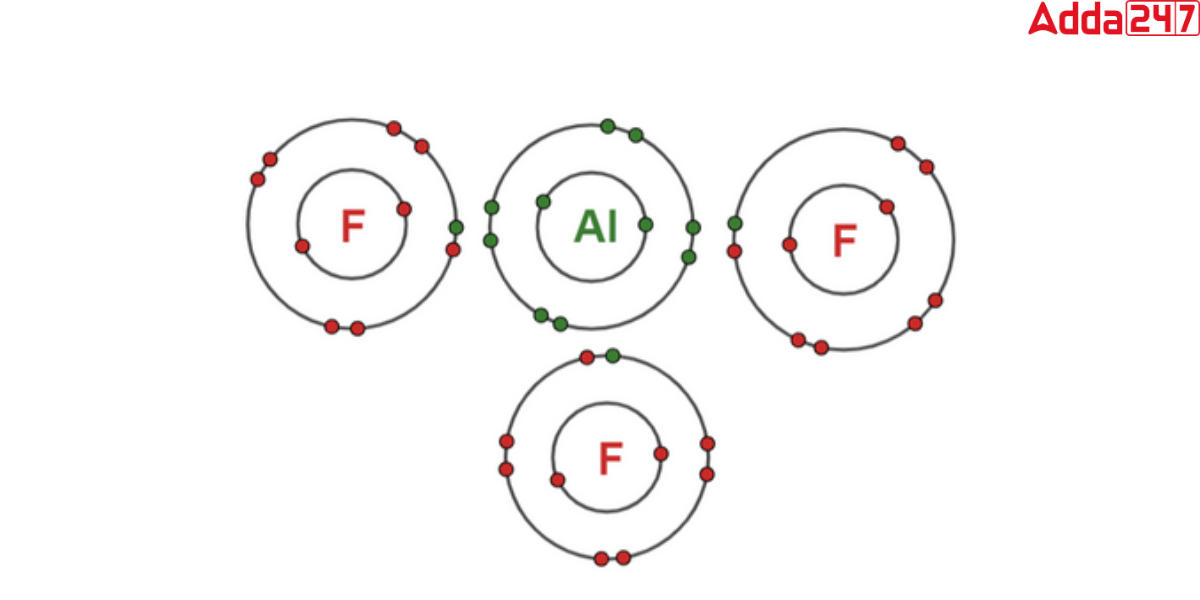
Each aluminium ion is surrounded by six fluoride ions, while each fluoride ion is surrounded by four aluminium ions. The aluminium ion (Al3+) in the aluminium fluoride formula has a charge of +3, indicating the loss of three electrons to establish a stable electron configuration. The fluoride ion (F) has a charge of -1, suggesting that it accepts an additional electron to achieve stability. The pairing of these oppositely charged ions assures the electrical neutrality of the molecule
Aluminium Fluoride Properties
Aluminium fluoride is a colourless chemical compound with the molecular formula AlF3.The appearance of luminium fluoride is white powder or granules. Explore the Physical and Chemical Properties of Aluminium Fluoride listed below.
Physical properties of Aluminium Fluoride
- Aluminium fluoride appears as white powder or granules.
- Aluminium fluoride is odourless.
- The melting point of aluminium fluoride is 1291 degrees Celsius.
It has a vapour pressure of 1 mm at 1238 °C. - Aluminium fluoride is not flammable in nature.
- It is soluble in water at a temperature of 25°C and has a solubility of approximately.559g/100 mL.
- When this chemical breaks down, it emits extremely dangerous hydrogen fluoride vapours.
- Aluminium fluoride is less affected by even highly concentrated acid.
- This molecule is also only weakly soluble in alkalis and acids.
The compound is less prone to oxidation as compared to trialkylaluminiums. - Aluminium fluoride sublimates at a pressure of 760 psi and a temperature of 1272 °C and a pressure of 760 mmHg.
Chemical Properties of Aluminium Fluoride
- It is a stable substance that dissolves readily in both water and hydrofluoric acid.
- An excellent heat and electrical conductor is aluminium. It has two thirds the electrical conductivity of copper and half the heat conductivity.
- Along with AlF3, there are numerous other aluminium fluoride hydrates. These substances also have the formula AlF3.xH2O, a monohydrate with x=1 and two polymorphs of a trihydrate with x=3, a hexahydrate with x=6 and a nonahydrate with x=9.
- When this substance reaches the point of breakdown, deadly hydrogen fluoride vapours are released.
Preparation of Aluminium Fluoride
Aluminium fluoride is found in nature as oskarssonite and rosenbergite. Although it can also be made commercially for Industry use. There are various ways to prepare the Aluminium fluoride in laberatory Condition.
- At 700 degrees Celsius, Aluminium fluoride is combined with alumina or aluminium oxide to produce of aluminium fluoride.
- Aluminium fluoride can also be made using hexafluorosilicic acid.H2[SiF6] + Al2O3 + 3 H2O → 2 AlF3 + SiO2 + 4 H2O
- Thermal breakdown of ammonium hexafluoroaluminate produces this compound.
- AlF3 can also be made on in laboratories by treating aluminium hydroxide or aluminium metal with hydrogen fluoride.
Aluminium Fluoride Uses
Aluminium fluoride has numerous applications in a variety of industries and activities. Aluminium fluoride has the following applications:
- Aluminium fluoride is used as an additive in aluminium production and electrolysis.
- It’s used in the fermentation process in breweries and wineries.
- It is an important component in the production of fluoroaluminate glass which is employed in a range of optical fibre applications, including medical and fiber-optic imaging.
- Aluminium fluoride is commonly utilised in ceramics as the major chemical constituent of finished items.
Aluminium Fluoride Compound Name
The chemical compound formed between aluminum and fluoride ions is called “aluminum fluoride.” Its chemical formula is AlF3.



 NTA NEET Exam Date 2025 OUT, Exam Timing...
NTA NEET Exam Date 2025 OUT, Exam Timing...
 NEET Admit Card 2025 Release Date by NTA
NEET Admit Card 2025 Release Date by NTA
 Anna University Result 2025 OUT at coe1....
Anna University Result 2025 OUT at coe1....










