Ammonia
With the formula NH3, ammonia is a nitrogen and hydrogen inorganic chemical. Ammonia, the simplest pnictogen hydride and a stable binary hydride is a colorless gas with a strong, pungent odor. It contributes considerably to the nutritional demands of terrestrial creatures by serving as a precursor to 45% of the world’s food and fertilizers. Biologically, it is a common nitrogenous waste, especially among aquatic animals. Around 70% of ammonia is used to create fertilizers, including urea and diammonium phosphate, in a variety of shapes and compositions. Moreover, pure ammonia is sprayed straight onto the ground.
Ammonia Formula
The ammonia formula is NH3. It is a compound made up of one nitrogen atom (N) and three hydrogen atoms (H). Ammonia is a colorless gas with a pungent odor and is commonly used in various industrial applications, as well as in household cleaning products and as a refrigerant.
Ammonia Symbol
ammonia (NH3), colorless, pungent gas composed of nitrogen and hydrogen.
Ammonia Uses
- Ammonia is also a key ingredient in many commercial cleaning solutions and is a building component for the manufacture of numerous medicinal medicines. It is mostly gathered through the displacement of both air and water downward.
- Although ammonia is widely used and found in nature on Earth and on the outer planets of the Solar System, it is dangerous and caustic when it is concentrated.
- Facilities that produce, store, or use it in considerable amounts are subject to severe reporting requirements because it is categorised as a very dangerous material in many nations.
- Ammonia was produced industrially on a global scale in 2018 in an amount of 175 million tonnes, which is essentially unchanged from the 175 million tonnes produced in 2013.
- This was 235 million tonnes in 2021, and hardly any of it was produced in the United States.
- Industrial ammonia is either carried in tank cars or cylinders or marketed as ammonia liquor that has been compressed or refrigerated.
The creation of ammonia from the elements hydrogen and nitrogen is challenging for fundamental reasons, requiring high pressures and high temperatures. The invention of the Haber process at the turn of the 20th century revolutionised agriculture and made it possible for industrial manufacturing.
Ammonia Occurrence
Ammonia is a chemical that is formed from nitrogenous plant and animal tissue and is present on Earth in minute amounts. Little amounts of ammonia and ammonium salts can also be found in rainfall, although ammonium chloride and ammonium sulphate are only found in volcanic areas. Guano from Patagonia has been found to contain ammonium bicarbonate crystals.
On smaller, icy bodies like Pluto, ammonia can act as a geologically significant antifreeze because a mixture of water and ammonia can have a melting point as low as 100 °C (148 °F; 173 K) if the ammonia concentration is high enough. This allows such bodies to retain internal oceans and active geology at a much lower temperature than would otherwise be possible. Ammonia is also found throughout the Solar System on Mars, Jupiter, Saturn, Ammoniacal substances are those that contain ammonia or are analogous to it.
Ammonia Properties
A colourless gas with a distinctively strong smell, ammonia. Due to its lower density (0.589 times that of air), it is lighter than air. Due to the molecules’ strong hydrogen bonds, it can be easily liquefied. Ammonia gas transforms into a colourless liquid that boils at 33.1 °C (27.58 °F) and crystallises into colourless ice at 77.7 °C (107.86 °F). At extremely high pressures and temperatures, such as supercritical conditions, there are not many data available.
In water, ammonia dissolves easily. It can be boiled out of an aqueous solution to remove it. Ammonia in aqueous solution is basic. Ammonia in water at its highest concentration has a density of 0.880 g/cm3.
Ammonia Chemical Properties
1. Ammonia is a basic chemical. Turmeric paper turns brown, damp red litmus paper turns blue, and phenolphthalein turns pink. In water, it dissolves to produce ammonium hydroxide.
NH3 + H2O ———-> NH4OH
2. It transforms into the matching salt when it interacts with acids like HCl, etc.
NH3 + HCl ———-> NH4Cl
3. Ammonia does not sustain burning and is not a flammable substance.
4. Ammonia burns in an oxygen environment with a greenish-yellow flame under the right circumstances, forming nitrogen and water in the process.
4NH3 + 3O2 ———-> 2N2 + 6H2O
5. Ammonia oxidises into nitrogen when it comes into contact with heated copper oxide.
2NH3 + 3CuO ———-> N2 + 3Cu + 3H2O
6. Nitric oxide is produced when ammonia and air or oxygen are combined and heated to 800-850°C in the presence of platinum gauze (NO).
4NH3 + 5O2 ———-> 4NO + 6H2O
7. When ammonia is heated to a temperature above 1100 °C or exposed to electric sparks, it splits into nitrogen and hydrogen.
8. Halogens can oxidise ammonia. Nitrogen is evolved when ammonia levels are excessive.
8NH3 + 3Cl2 ————-> N2 + 6NH4Cl
9. Nitrogen trichloride, a substance that is highly explosive, is created when chlorine levels are too high.
NH3 + 3Cl2 ————-> NCl3 + 3HCl
Ammonia Physical Properties
Following are the Physical Properties of Ammonia:
- A colourless gas with a distinctively strong smell, ammonia. It tastes sour and scorching.
- It is air-less in weight.
- The solution is of a basic character and extremely soluble in water.
- At 0°C and 760 mm, one volume of water dissolves 1300 litres of ammonia.
- As with HCl gas, the Fountain Experiment can be used to show the high solubility of ammonia in water.
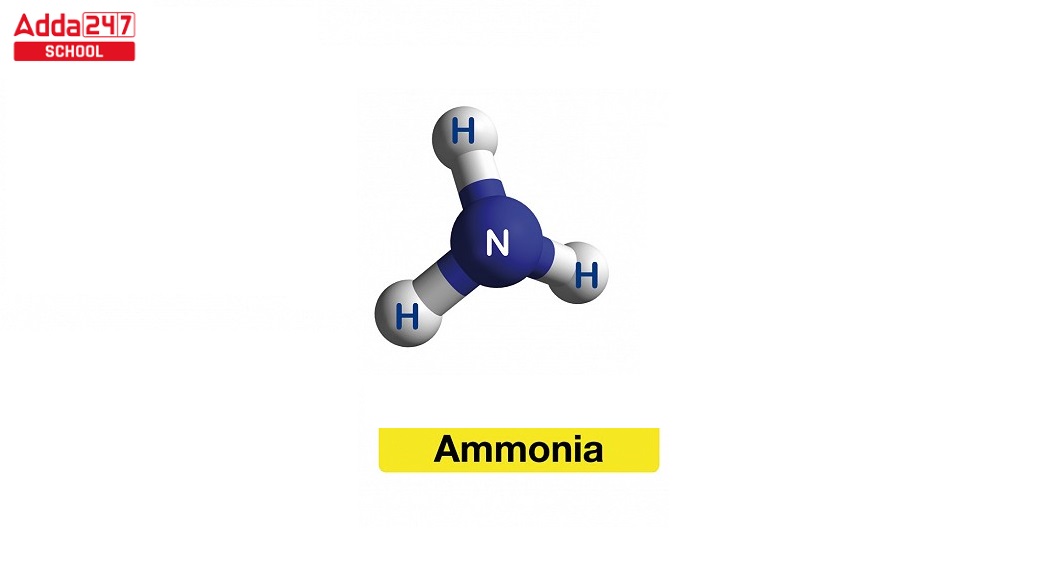
Ammonia Structure
- According to the valence shell electron pair repulsion theory (VSEPR theory), the ammonia molecule has a trigonal pyramidal form with an empirically determined bond angle of 106.7°.
- With an additional electron from each hydrogen atom, the central nitrogen atom possesses six outside electrons in total. This results in four pairs of tetrahedrally organised electrons, or eight total electrons.
- One lone pair of electrons remains after three of these electron pairs are employed as bond pairs.
- The bond angle is not 109.5°, as expected for a typical tetrahedral structure, but rather 106.8° because the lone pair repels more strongly than bond pairs.
- The molecule becomes polar due to this shape’s dipole moment.
- Ammonia is extremely miscible with water due to the polarity of the molecule and, in particular, its capacity to form hydrogen bonds.
- Ammonia becomes a base and a proton acceptor due to the lone pair.
- Ammonia is moderately basic; a 1.0 M aqueous solution has a pH of 11.6, and 99.4% of the ammonia molecules are protonated if a strong acid is added to the solution until it is neutral (pH = 7).
- The quantity of ammonium [NH4]+ is influenced by temperature and salinity as well.
- The latter is isoelectronic with methane and has the form of a standard tetrahedron.
Mercury Metal- Uses, Formula, Colour, Density, Properties
Slaked Lime Formula and Chemical Name, Uses









 CUET History Syllabus 2026 (Updated), Do...
CUET History Syllabus 2026 (Updated), Do...
 CUET General Test Syllabus 2026 (Latest)...
CUET General Test Syllabus 2026 (Latest)...
 CUET Economics Syllabus 2026, Exam Patte...
CUET Economics Syllabus 2026, Exam Patte...














