The atomic mass of an element is the average mass of its atoms measured in atomic mass units (amu, commonly known as daltons, D). atomic mass is also known as atomic weight, but the term “mass” is more preciseThe atomic mass is a weighted average of all the isotopes of that element, calculated by multiplying the mass of each isotope by its abundance. (A.) In this article, we will learn about the Atomic Mass of Elements 1 to 30 with Symbols.
Atomic Mass of Elements
The absolute mass of a single atom is its atomic mass, which is measured in atomic mass units, or amu. Carbon-12, for example, is a typical carbon atom with six neutrons and six protons. It has a mass of 12 atomic units. In most cases, the atomic mass number is rounded to the next whole number. Because the isotopes of an element have different atomic masses, researchers can determine the element’s overall atomic mass (also known as the atomic weight). The normal of the atomic masses of the seeming plurality of distinct isotopes in an example is the general atomic mass. The amount of example an isotope makes up determines how much it contributes to the normal. Overall atomic masses are determined for naturally occurring isotopes of each element, weighted by the weight of those individual isotopes on Earth, as shown in periodic tables like the one for hydrogen.
Atomic Mass of Elements Formula
The sum of the masses of protons, neutrons, and electrons in an atom or group of atoms is called atomic mass. Because electrons have a very low mass compared to protons or neutrons, their mass has no bearing on the calculation. The average mass of the naturally occurring isotopes of an element in comparison to the mass of an atom of 12C is known as relative atomic mass. This means that one atom has an atomic mass of exactly 12. The mass of an atom is not expressed in units. The atomic mass formula is shown below.
Atomic mass is the sum of protons, neutrons, and electrons. Hence,
| Atomic mass = Mass of protons + Mass of neutrons + Mass of electrons |
Atomic Mass of First 30 Elements with Symbols
Atomic mass is the mass of an atom. It is typically expressed in atomic mass units (amu), which are defined as 1/12 of the mass of a carbon-12 atom. The atomic mass of an atom is a weighted average of the masses of the isotopes of that atom. Isotopes are atoms of the same element that have different numbers of neutrons. For example, carbon has three isotopes: carbon-12, carbon-13, and carbon-14. Carbon-12 has 6 protons and 6 neutrons, carbon-13 has 6 protons and 7 neutrons, and carbon-14 has 6 protons and 8 neutrons.
First 30 Elements with symbols
The elements from 1 to 30 in the periodic table are as follows:
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Neon (Ne)
- Sodium (Na)
- Magnesium (Mg)
- Aluminum (Al)
- Silicon (Si)
- Phosphorus (P)
- Sulfur (S)
- Chlorine (Cl)
- Argon (Ar)
- Potassium (K)
- Calcium (Ca)
- Scandium (Sc)
- Titanium (Ti)
- Vanadium (V)
- Chromium (Cr)
- Manganese (Mn)
- Iron (Fe)
- Cobalt (Co)
- Nickel (Ni)
- Copper (Cu)
- Zinc (Zn)
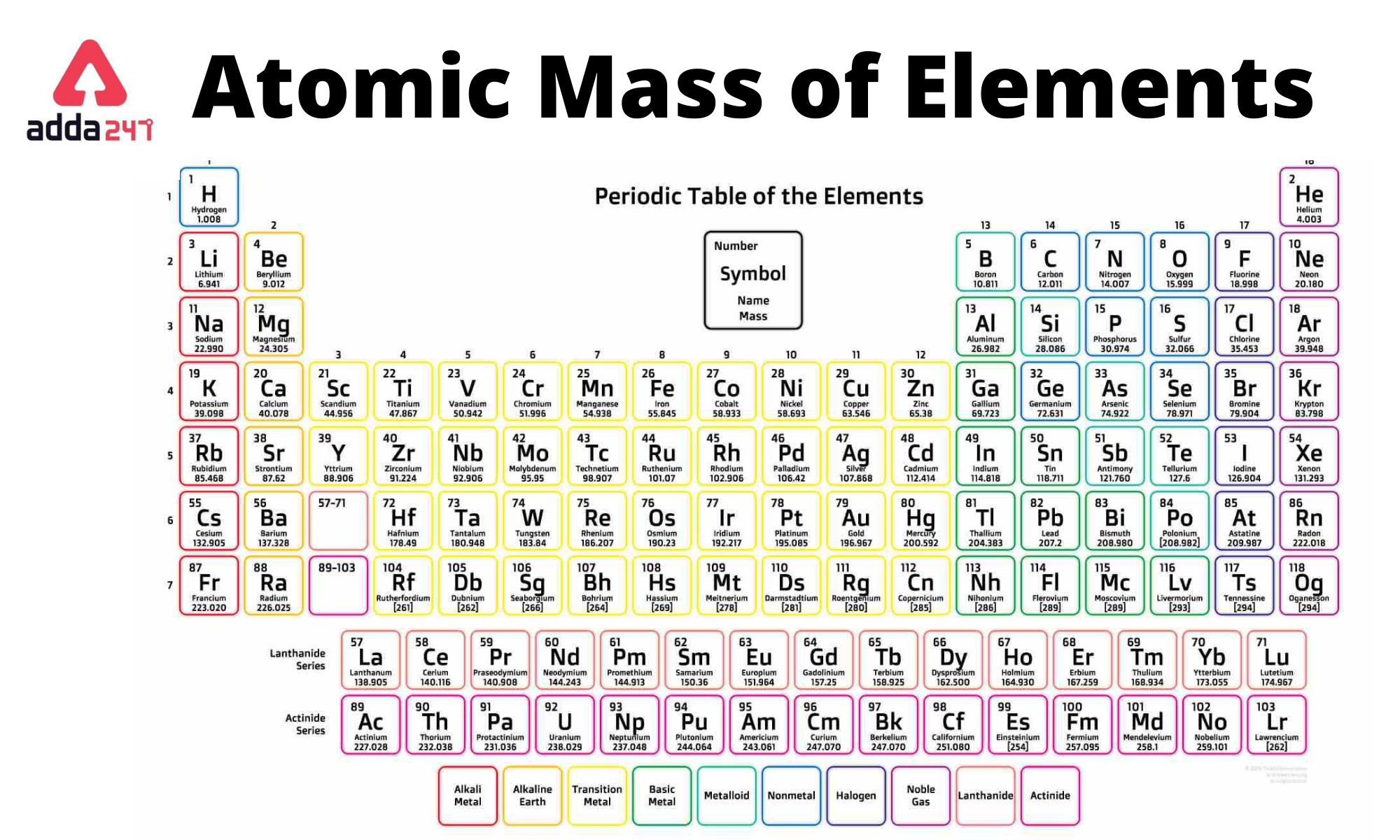
Periodic Table With Atomic Mass
list of the elements in the periodic table along with their atomic symbols and approximate atomic masses. Please note that atomic masses can vary slightly depending on the specific isotope of an element. The values provided here are approximate and are based on the most common isotope for each element.
- Hydrogen (H) – 1.008
- Helium (He) – 4.0026
- Lithium (Li) – 6.94
- Beryllium (Be) – 9.0122
- Boron (B) – 10.81
- Carbon (C) – 12.011
- Nitrogen (N) – 14.007
- Oxygen (O) – 15.999
- Fluorine (F) – 18.998
- Neon (Ne) – 20.180
- Sodium (Na) – 22.990
- Magnesium (Mg) – 24.305
- Aluminum (Al) – 26.982
- Silicon (Si) – 28.085
- Phosphorus (P) – 30.974
- Sulfur (S) – 32.06
- Chlorine (Cl) – 35.453
- Argon (Ar) – 39.948
- Potassium (K) – 39.098
- Calcium (Ca) – 40.078
- Scandium (Sc) – 44.956
- Titanium (Ti) – 47.867
- Vanadium (V) – 50.942
- Chromium (Cr) – 51.996
- Manganese (Mn) – 54.938
- Iron (Fe) – 55.845
- Cobalt (Co) – 58.933
- Nickel (Ni) – 58.693
- Copper (Cu) – 63.546
- Zinc (Zn) – 65.38
- Gallium (Ga) – 69.723
- Germanium (Ge) – 72.63
- Arsenic (As) – 74.922
- Selenium (Se) – 78.971
- Bromine (Br) – 79.904
- Krypton (Kr) – 83.798
- Rubidium (Rb) – 85.468
- Strontium (Sr) – 87.62
- Yttrium (Y) – 88.906
- Zirconium (Zr) – 91.224
- Niobium (Nb) – 92.906
- Molybdenum (Mo) – 95.95
- Technetium (Tc) – [98]*
- Ruthenium (Ru) – 101.1
- Rhodium (Rh) – 102.9
- Palladium (Pd) – 106.4
- Silver (Ag) – 107.9
- Cadmium (Cd) – 112.4
- Indium (In) – 114.8
- Tin (Sn) – 118.7
- Antimony (Sb) – 121.8
- Tellurium (Te) – 127.6
- Iodine (I) – 126.9
- Xenon (Xe) – 131.3
- Cesium (Cs) – 132.9
- Barium (Ba) – 137.3
- Lanthanum (La) – 138.9
- Cerium (Ce) – 140.1
- Praseodymium (Pr) – 140.9
- Neodymium (Nd) – 144.2
- Promethium (Pm) – [145]*
- Samarium (Sm) – 150.4
- Europium (Eu) – 152.0
- Gadolinium (Gd) – 157.3
- Terbium (Tb) – 158.9
- Dysprosium (Dy) – 162.5
- Holmium (Ho) – 164.9
- Erbium (Er) – 167.3
- Thulium (Tm) – 168.9
- Ytterbium (Yb) – 173.0
- Lutetium (Lu) – 175.0
- Hafnium (Hf) – 178.5
- Tantalum (Ta) – 180.9
- Tungsten (W) – 183.8
- Rhenium (Re) – 186.2
- Osmium (Os) – 190.2
- Iridium (Ir) – 192.2
- Platinum (Pt) – 195.1
- Gold (Au) – 197.0
- Mercury (Hg) – 200.6
- Thallium (Tl) – 204.4
- Lead (Pb) – 207.2
- Bismuth (Bi) – 208.9
- Polonium (Po) – [209]*
- Astatine (At) – [210]*
- Radon (Rn) – [222]*
- Francium (Fr) – [223]*
- Radium (Ra) – [226]*
- Actinium (Ac) – [227]*
- Thorium (Th) – 232.0
- Protactinium (Pa) – 231.0
- Uranium (U) – 238.0
- Neptunium (Np) – [237]*
- Plutonium (Pu) – [244]*
- Americium (Am) – [243]*
- Curium (Cm) – [247]*
- Berkelium (Bk) – [247]*
- Californium (Cf) – [251]*
- Einsteinium (Es) – [252]*
- Fermium (Fm) – [257]*
- Mendelevium (Md) – [258]*
- Nobelium (No) – [259]*
- Lawrencium (Lr) – [266]*
- Rutherfordium (Rf) – [267]*
- Dubnium (Db) – [270]*
- Seaborgium (Sg) – [271]*
- Bohrium (Bh) – [270]*
- Hassium (Hs) – [277]*
- Meitnerium (Mt) – [276]*
- Darmstadtium (Ds) – [276]
These are the first 30 elements in the periodic table, listed in order of increasing atomic number.
1 to 30 Elements with Symbols and Atomic mass
Following is the periodic table of 1 to 30 elements with symbols.
| Atomic Number – Name of Element | Symbol | Atomic Mass of Element (amu) |
| 1 – Hydrogen | H | 1.00 |
| 2 – Helium | He | 4.00 |
| 3 – Lithium | Li | 6.94 |
| 4 – Beryllium | Be | 9.01 |
| 5 – Boron | B | 1.081 |
| 6 – Carbon | C | 12.01 |
| 7 – Nitrogen | N | 14.00 |
| 8 – Oxygen | O | 15.99 |
| 9 – Fluorine | F | 18.99 |
| 10 – Neon | Ne | 20.17 |
| 11 – Sodium | Na | 22.98 |
| 12 – Magnesium | Mg | 24.30 |
| 13 – Aluminum | Al | 26.98 |
| 14 – Silicon | Si | 28.08 |
| 15 – Phosphorous | P | 30.97 |
| 16 – Sulphur | S | 32.06 |
| 17 – Chlorine | Cl | 35.45 |
| 18 – Argon | Ar | 39.94 |
| 19 – Potassium | K | 39.09 |
| 20 – Calcium | Ca | 40.08 |
| 21 – Scandium | Sc | 44.95 |
| 22 – Titanium | Ti | 47.90 |
| 23 – Vanadium | V | 50.94 |
| 24 – Chromium | Cr | 51.99 |
| 25 – Manganese | Mn | 54.93 |
| 26 – Iron | Fe | 55.84 |
| 27 – Cobalt | Co | 58.93 |
| 28 – Nickel | Ni | 58.70 |
| 29 – Copper | Cu | 63.54 |
| 30 – Zinc | Zn | 65.38 |
Atomic Mass Table of the first 20 elements
Following is the Table of the atomic mass of the first 20 elements of the periodic table:
| Atomic Number – Name of Element | Symbol | Atomic Mass of Element (amu) |
| 1 – Hydrogen | H | 1.00 |
| 2 – Helium | He | 4.00 |
| 3 – Lithium | Li | 6.94 |
| 4 – Beryllium | Be | 9.01 |
| 5 – Boron | B | 1.081 |
| 6 – Carbon | C | 12.01 |
| 7 – Nitrogen | N | 14.00 |
| 8 – Oxygen | O | 15.99 |
| 9 – Fluorine | F | 18.99 |
| 10 – Neon | Ne | 20.17 |
| 11 – Sodium | Na | 22.98 |
| 12 – Magnesium | Mg | 24.30 |
| 13 – Aluminum | Al | 26.98 |
| 14 – Silicon | Si | 28.08 |
| 15 – Phosphorous | P | 30.97 |
| 16 – Sulphur | S | 32.06 |
| 17 – Chlorine | Cl | 35.45 |
| 18 – Argon | Ar | 39.94 |
| 19 – Potassium | K | 39.09 |
| 20 – Calcium | Ca | 40.08 |
Atomic Mass of all Elements PDF
The atomic Mass of Elements 1 to 30 with Symbols is essential for the students for the board. Here Adda247 team provides you atomic Mass of Elements 1 to 30 PDF so that students can download and keep it for future reference.Check out the atomic Mass of Elements 1 to 30 with Symbols PDf below.
Download the Periodic Table with Atomic Mass of Elements 1 to 30 with Symbols PDF








 CUET PG 2026 Registration Form OUT at ex...
CUET PG 2026 Registration Form OUT at ex...
 AILET Result 2026 Release Date, How to D...
AILET Result 2026 Release Date, How to D...
 CLAT Result 2026 Out, Download Scorecard...
CLAT Result 2026 Out, Download Scorecard...














