The Aufbau principle governs how electrons are arranged in the atomic orbitals of an atom when it is in its lowest energy state. Electrons are arranged in atomic orbitals based on their energy levels, starting from the lowest to the highest. The Aufbau principle states that atomic orbitals with lower energy levels are filled before those with higher energy levels. It is one of the most important concepts asked in the chemistry exam. It is also very important in general to know this principle so that you can understand the electronic nature of any element.
Aufbau Principle
The Aufbau principle, sometimes known as the Aufbau rule, asserts that when an atom or ion is in its ground state, electrons occupy subshells with the lowest possible energy first, then subshells with higher energy. According to this Principle The 1s subshell, for example, is occupied before the 2s subshell. An atom’s or ion’s electrons form the most stable electron configuration feasible in this fashion. The phosphorus atom, for Aufbau Principle example, has the configuration 1s2 2s2 2p6 3s2 3p3, indicating that the 1s subshell possesses two electrons, and so on.
What is Aufbau Principle?
The Aufbau principle, also known as the “building-up principle,” is a fundamental concept in chemistry that describes the order in which electrons fill the energy levels, or orbitals, of an atom. It’s used to predict the electron configuration of atoms, which is the arrangement of electrons in various energy levels around the nucleus of an atom.
According to the Aufbau principle:
- Electrons occupy the lowest energy orbitals available first.
- Orbitals are filled in order of increasing energy, starting with the lowest energy level (the 1s orbital) and progressing to higher energy levels.
In simpler terms, electrons will fill the orbitals in a way that minimizes their energy. The Aufbau principle provides a systematic way to determine the electron configuration of an atom by following the sequence of orbitals in order of increasing energy.
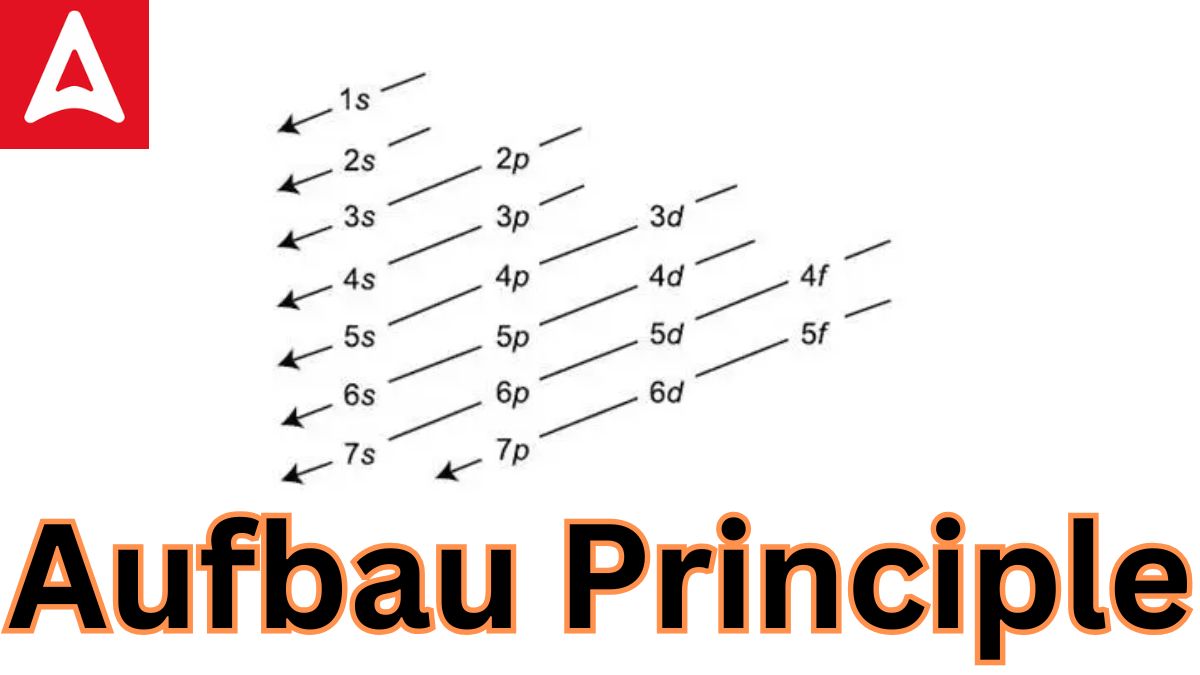
The order in which orbitals are filled is as follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, and so on.
This principle is a key foundation in understanding the periodic table and the arrangement of elements based on their electron configurations. It helps explain why certain elements exhibit similar chemical properties and trends across the periodic table.
Aufbau Principle Definition
The Aufbau principle can be used to figure out where electrons are in an atom and what energy levels they correspond to. Carbon, for example, has six electrons and has the electrical structure 1s²2s²2p².
The Aufbau Principle is worth noting that each orbital can only store two electrons (according to Pauli’s exclusion principle). In addition, the way electrons are filled into orbitals in a single subshell must adhere to Hund’s rule, which states that every orbital in a given subshell must be single-occupied by electrons before any two electrons pair up in an orbital.
Aufbau Principle Formula
The Aufbau principle is a fundamental concept in chemistry that describes the order in which electrons fill atomic orbitals in an atom. It states that electrons will occupy the lowest energy orbitals available before moving to higher energy ones. This principle is used to determine the electron configuration of atoms.
Aufbau Principle Formula Example
Aufbau Principle formula is= 1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,4f,5d,6p,7s…
The formula or sequence derived from the Aufbau principle explained as follows:
- Electrons fill the lowest energy level orbitals first.
- Within a given energy level (shell), electrons fill the orbitals in the following order of increasing energy:1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
Where:
- The numbers (1, 2, 3, …) represent the principal quantum number, indicating the energy level or shell of the electron.
- The letters (s, p, d, f) represent the orbital type (spherical, dumbbell, double dumbbell, and complex shapes, respectively).
Keep in mind that this sequence represents the order in which orbitals are filled as you move from lighter to heavier elements in the periodic table. Exceptions can occur due to electron-electron interactions and the stability gained from having fully or half-filled orbitals. For example, chromium and copper are exceptions to the Aufbau principle due to the stability gained by having a half-filled or fully filled d subshell, respectively.
Aufbau Principle Class 11
The Aufbau principle, also known as the “building-up” principle, is a fundamental concept in chemistry that explains how electrons are distributed within the electron shells or energy levels of an atom. This principle helps to determine the electronic configuration of an atom and how the electrons fill up the available energy levels.
In the context of class 11 chemistry, the Aufbau principle is typically introduced as part of the study of atomic structure and the periodic table. Here’s a breakdown of the key points:
- Sublevels and Orbitals: Electrons within an atom are arranged in energy levels (also known as shells) and sublevels (s, p, d, f). Each sublevel consists of one or more orbitals, which are regions of space where electrons are likely to be found.
- Order of Filling: According to the Aufbau principle, electrons fill the lowest energy levels and sublevels first before filling higher energy levels. This means that the 1s sublevel (lowest energy) is filled before the 2s sublevel, which is then followed by the 2p sublevel, and so on.
- Pauli Exclusion Principle: This principle states that each orbital can hold a maximum of two electrons with opposite spins. In other words, if two electrons occupy the same orbital, they must have opposite spins (one “up” and one “down”).
- Hund’s Rule: Hund’s rule states that when filling degenerate (same energy) orbitals (such as the three p orbitals in a given p sublevel), electrons will first occupy separate orbitals with parallel spins before pairing up. This minimizes electron-electron repulsion and stabilizes the atom.
Here’s the order of filling based on the Aufbau principle:
- 1s
- 2s
- 2p
- 3s
- 3p
- 4s
- 3d
- 4p
- 5s
- 4d
- 5p
- 6s
- 4f
- 5d
- 6p
- 7s
- 5f
- 6d
- 7p
Keep in mind that this principle helps to understand the general order of electron filling, but there are exceptions in some transition metal elements due to the intricate energy levels and sublevels involved.
Understanding the Aufbau principle is crucial in predicting the chemical properties of elements, explaining trends in the periodic table, and comprehending how atoms form ions and bond with each other.
Aufbau Principle Limitations for Class 11
Chromium’s electron configuration is [Ar]3d54s1, not [Ar]3d44s2 (as suggested by the Aufbau principle). This is due to a number of variables, including half-filled subshells’ enhanced stability and the comparatively small energy gap between the 3d and 4s subshells.
Lower electron-electron repulsions in the orbitals of half-filled subshells increase stability through reducing electron-electron repulsions. Similarly, totally filled subshells improve the atom’s stability. As a result, some atoms’ electron configurations defy the Aufbau principle (depending on the energy gap between the orbitals).
Aufbau Principle with Examples
Students can understand the Aufbau rule in detail by going through the following example points.
- The Aufbau principle states that electrons will inhabit the lowest-energy orbitals first. This means that electrons can only enter higher-energy orbitals after lower-energy orbitals have been entirely filled.
- The (n+l) rule can be used to establish the sequence in which the energy of orbitals grows, with the sum of the primary and azimuthal quantum numbers determining the orbital’s energy level.
- Lower orbital energies correspond to lower (n+l) values. When two orbitals have the same (n+l) values, the orbital with the lower n value is said to have less energy.
- 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on are the orders in which the orbitals are filled with electrons.
Aufbau Principle Example: Sulphur
From Aufbau Principle we are writing the Electron Configuration of Sulphur
- Sulphur has an atomic number of 16, indicating that it has a total of 16 electrons.
- According to the Aufbau principle, two of these electrons are in the 1s subshell, eight are in the 2s and 2p subshells, and the rest are dispersed between the 3s and 3p subshells.
- As a result, sulphur’s electron configuration can be expressed as 1s22s22p63s23p4.
Aufbau Principle Examples for Class 11
The Aufbau principle, also known as the building-up principle, is a fundamental concept in quantum mechanics that explains how electrons are arranged in an atom’s energy levels or electron shells. According to the Aufbau principle, electrons fill the lowest energy levels first before moving to higher energy levels. Here are some examples to illustrate the Aufbau principle:
- Hydrogen (H):
- Hydrogen has one electron.
- The electron occupies the lowest energy level, which is the first shell (n=1).
- Helium (He):
- Helium has two electrons.
- The first electron occupies the first shell (n=1), and the second electron goes to the same shell because it’s the lowest energy level available.
- Lithium (Li):
- Lithium has three electrons.
- Two electrons fill the first shell (n=1), and the third electron goes to the second shell (n=2) since it has a slightly higher energy level.
- Carbon (C):
- Carbon has six electrons.
- Two electrons fill the first shell (n=1), and the remaining four electrons start filling the second shell (n=2).
- Oxygen (O):
- Oxygen has eight electrons.
- Two electrons fill the first shell (n=1), and the remaining six electrons continue filling the second shell (n=2).
- Nitrogen (N):
- Nitrogen has seven electrons.
- Two electrons fill the first shell (n=1), and the remaining five electrons start filling the second shell (n=2).
- Neon (Ne):
- Neon has ten electrons.
- Two electrons fill the first shell (n=1), and the remaining eight electrons fill the second shell (n=2).
- Sodium (Na):
- Sodium has eleven electrons.
- Two electrons fill the first shell (n=1), and the remaining nine electrons start filling the second shell (n=2).
The pattern continues as elements become more complex, and electrons continue to fill higher energy levels based on the Aufbau principle. It’s important to note that there are exceptions to this principle in transition metals due to the intricacies of electron orbital energies, electron-electron repulsions, and other factors.
Aufbau Rule
The Aufbau Rule, also known as the “building-up” principle, is a fundamental concept in chemistry that describes how electrons fill the atomic orbitals of an atom in a specific order as the atomic number increases. It’s a crucial part of understanding the electronic structure of atoms and the arrangement of electrons within them.
Aufbau Rule: Key Points
- Orbital Filling Order: Electrons occupy the lowest energy orbitals available to them before filling higher energy orbitals.
- Energy Levels and Sublevels: Electrons fill orbitals according to their energy levels and sublevels. The energy levels are represented by the principal quantum number (n), and the sublevels are represented by letters (s, p, d, f).
- Pauli Exclusion Principle: Each atomic orbital can hold a maximum of two electrons with opposite spins. This means that if two electrons occupy the same orbital, they must have opposite spins.
- Hund’s Rule: When electrons are filling degenerate (same energy level) orbitals, they prefer to occupy separate orbitals with parallel spins before pairing up.
The filling order for the first few energy levels and sublevels according to the Aufbau principle is as follows:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 4f¹⁴ 5s² 5p⁶ 5d¹⁰ 5f¹⁴ …
For example, consider the electron configuration of carbon (atomic number 6):
Carbon has 6 electrons. Following the principle, the electrons are arranged as follows: 1s² 2s² 2p²
- The first two electrons occupy the 1s orbital.
- The next four electrons fill the 2s and 2p orbitals.
- The 2p orbital is split into three degenerate (same energy) orbitals (2pₓ, 2pᵧ, 2pₓz), and each of these orbitals initially gets one electron, all with parallel spins (Hund’s rule).
This electron configuration reflects the Aufbau principle by filling the lowest energy orbitals first and adhering to the Pauli exclusion principle and Hund’s rule.
In summary, the principle is a foundational concept in understanding the arrangement of electrons in atoms, guiding the order in which electrons fill orbitals based on energy levels and sublevels.
Aufbau Principle for Ground State of Multi-electron Atoms/Ions
Students are often asked to explain Aufbau Principle for The Ground State of Multi-electron Atoms/Ions. According to Aufbau Principle, the ground state electron configuration in atoms/ions with two or more electrons
(1) Minimises the total energy of the electrons
(2) Follows the Pauli exclusion principle
(3) Follows the Hunds rule of maximum multiplicity
(4) Takes the exchange interaction into account
Related Post:
- Area Of Sphere- Formula In Maths
- BODMAS Full Form With Sign, Examples In Computer
- Rank Of The Matrix – Definition, Formulas, Examples
- Electrochemical Series Of Metals- Ncert Trick, Table
- Redox Reaction- Definition, Examples, Balancing
- Who Invented Electricity In The World?
- Who Invented Exams?
- NCC Full Form In Army, School, And College
- What Is The Valency Of Nitrogen?










 Chemistry Investigatory Project Class 12...
Chemistry Investigatory Project Class 12...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...
 CUET Chemistry Syllabus 2026, Download O...
CUET Chemistry Syllabus 2026, Download O...














