Table of Contents
Beer Lambert Law: The Beer Lambert Law is used to define the relationship between the intensity of visible UV light and the precise amount of substance present. Beer Lambert Law’s derivation has numerous applications in current science. Used in modern laboratories for drug testing, organic chemistry, and quantification tests. These are some of the areas where this Beer Lambert Law can be applied.
Beer Lambert Law
The Beer Lambert Law, commonly known as Beer’s law, describes the link between light attenuation and the qualities of a substance. The absorbance of a solution is directly proportional to the concentration of the absorbing species and the path length of the light through the solution, according to the law. Beer Lambert Law equation is as follows:
A = εlc
The Beer Lambert law is a fundamental law of spectroscopy and is used in a wide variety of applications, including:
- Chemical concentration measurement in solutions
- Identifying chemical substances
- Molecular structure investigation
- Chemical reaction monitoring
The Beer Lambert law is an extremely useful tool for scientists and engineers, with several applications.
Beer Lambert Law Formula
The Beer Lambert law describes the relationship between a solution’s absorbance, the concentration of the absorbing species, and the path length of light through the solution. The Beer Lambert equation is as follows:
A = εlc
where:
A is the absorbance of the solution, which is a measure of how much light is absorbed by the solution.
ε is the molar extinction coefficient, which is a property of the absorbing species and is a measure of how strongly the species absorbs light.
l is the path length of the light through the solution, in centimeters.
c is the concentration of the absorbing species, in moles per liter.
Beer Lambert Law Equations Derivation
The Beer Lambert Law, often known as Beer’s Law, describes the relationship between a solution’s solute concentration and the amount of light absorbed. In spectrophotometry, it is widely used to calculate the concentration of an absorbing species in a sample.
The Beer Lambert Law has the following general equation:
A = ε * c * l
Where A = Solution Absorbance (unitless)
(Epsilon) = Molar absorptivity or molar extinction coefficient (Lmol1cm1) – a constant that varies according to the absorbing species and the wavelength of light employed.
c = Solute concentration (mol/L or M)
l = Path length or optical path length (cm) – the distance travelled by light through the solution.
The Beer Lambert Law is illustrated schematically below:
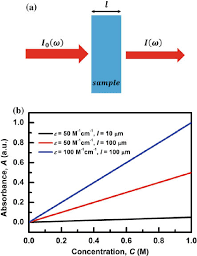
This figure shows:
- The light source produces a single wavelength of monochromatic light.
- The light passes through the absorbing species-containing sample.
- After the light has gone through the sample, the detector evaluates its intensity.
- The difference between the initial intensity and the intensity after passing through the sample is used to compute the solution’s absorbance (A).
- According to the Beer Lambert Law, absorbance (A) is directly proportional to the concentration (c) of the absorbing species and the route length (l), with the proportionality constant being the species’ molar absorptivity () at a given wavelength.
Application of Beer Lambert Law
The Beer Lambert law is a fundamental law of spectroscopy that has many applications. Among the most common Beer-Lambert law uses are:
- Chemical concentration in solutions is measured. One of the most common applications of the Beer-Lambert law is this. A solution’s absorbance can be used to calculate the concentration of the absorbing species. This is an extremely strong analytical chemistry instrument that is utilised in a variety of industries, including food, pharmaceutical, and environmental testing.
- Identifying chemical substances. The Beer-Lambert law can also be used to determine chemical identification. This is accomplished by comparing a solution’s absorbance to the absorbance of a known solution. This method is frequently employed in forensic chemistry and environmental analysis.
- Molecular structure research. The Beer Lambert law can also be used to investigate molecular structure. This is accomplished by measuring the absorbance of a solution at various wavelengths. A molecule’s absorption spectrum can reveal information about its molecular structure, such as the existence of functional groups.
- Chemical reactions are being monitored. Chemical reactions can also be monitored using the Beer-Lambert law. This is accomplished by monitoring the absorbance of a solution over time. The change in absorbance can be used to monitor the reaction’s progress.
The Beer Lambert law is a versatile tool that is utilized in a variety of situations. It is a fundamental spectroscopic law that is required for many analytical procedures.
Here are some further specific instances of how the Beer Lambert law is applied in various fields:
- The Beer Lambert law is used in the food business to determine the concentration of food additives such as vitamins and minerals. It is also used to check the freshness of foods like milk and wine.
- The Beer Lambert law is used in the pharmaceutical industry to determine the concentration of medicines in blood and other fluids. It is also used to assess the safety and efficacy of existing medications and to develop new ones.
- The Beer Lambert law is used in environmental monitoring to determine the concentration of contaminants in air and water. It is also used to monitor the environmental effects of pollution.
Beer Lambert Law Examples
Question: A solution of copper sulfate (CuSO4) has an absorbance of 0.75 when measured in a spectrophotometer at a wavelength of 450 nm. The path length of the cuvette is 1 cm. The molar absorptivity of CuSO4 at 450 nm is 0.0042 L·mol⁻¹·cm⁻¹. Calculate the concentration of copper sulfate in the solution.
Solution: Given: Absorbance (A) = 0.75 Path length (l) = 1 cm Molar absorptivity (ε) = 0.0042 L·mol⁻¹·cm⁻¹
We can use the Beer Lambert Law equation:
A = ε * c * l
To find the concentration (c), rearrange the equation:
c = A / (ε * l)
Now, plug in the values:
c = 0.75 / (0.0042 L·mol⁻¹·cm⁻¹ * 1 cm)
c ≈ 0.75 / 0.0042
c ≈ 178.57 mol/L
The concentration of copper sulfate in the solution is approximately 178.57 mol/L (or 178.57 M).
Question: You are given a solution of a compound with an unknown concentration. You measure its absorbance in a spectrophotometer at a specific wavelength of 520 nm. The absorbance of the solution is found to be 0.45, and the path length of the cuvette used in the spectrophotometer is 2 cm. The molar absorptivity of the compound at 520 nm is 0.0035 L·mol⁻¹·cm⁻¹. Calculate the concentration of the unknown compound in the solution.
Solution: Given: Absorbance (A) = 0.45 Path length (l) = 2 cm Molar absorptivity (ε) = 0.0035 L·mol⁻¹·cm⁻¹
Using the Beer Lambert Law equation:
A = ε * c * l
We can rearrange the equation to solve for the concentration (c):
c = A / (ε * l)
Now, plug in the values:
c = 0.45 / (0.0035 L·mol⁻¹·cm⁻¹ * 2 cm)
c ≈ 0.45 / 0.007
c ≈ 64.29 mol/L
The concentration of the unknown compound in the solution is approximately 64.29 mol/L (or 64.29 M).


 CUET UG Date Sheet 2025 @cuet.nta.nic.in...
CUET UG Date Sheet 2025 @cuet.nta.nic.in...
 UP, MP, CBSE Board Result 2025 Live Upda...
UP, MP, CBSE Board Result 2025 Live Upda...
 NTA NEET Cut Off 2025, Expected Cutoff f...
NTA NEET Cut Off 2025, Expected Cutoff f...




