CO- Carbon Monoxide
CO stands for carbon monoxide. Carbon monoxide is a colorless, odorless, and tasteless gas that consists of one carbon atom and one oxygen atom, chemically represented as CO. It is produced when carbon-containing fuels such as wood, gasoline, natural gas, and coal are burned with insufficient oxygen. Carbon monoxide is toxic to humans and animals because it can interfere with the body’s ability to transport oxygen in the bloodstream when it is inhaled in high concentrations.
Exposure to high levels of carbon monoxide can lead to carbon monoxide poisoning, which can cause symptoms such as headaches, dizziness, nausea, confusion, and, in severe cases, can be fatal. It is important to have carbon monoxide detectors in homes and other places where fuel-burning appliances are used to detect any leaks and prevent accidental poisoning.
Bond Order of CO
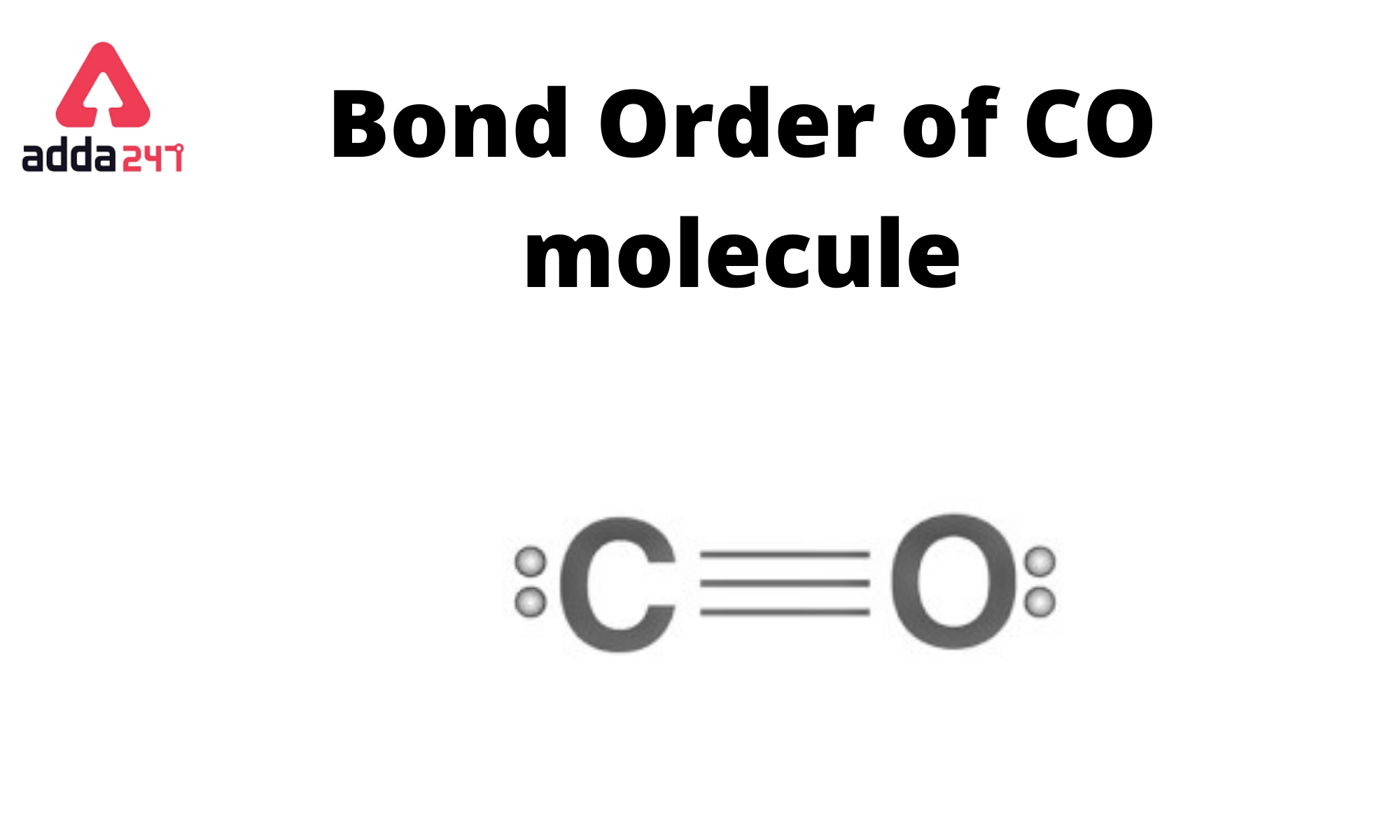
CO has a bond order of three (3). The bond order indicates how many chemical bonds exist between two atoms. Half of the difference between the number of electrons in bonding and antibonding orbitals is the Bond Order Formula.
What is Bond Order?
Bond order is defined in chemistry as the difference between the number of bonds and anti-bonds, as established by Linus Pauling. The number of electron pairs (covalent bonds) between two atoms is the bond number. For example, the bond number in diatomic nitrogen NN is 3, the bond number between the two carbon atoms in ethyne HCCH is 3, and the CH bond order is 1. A bond’s stability is determined by its bond number. The bond number of isoelectronic species is the same. Bond number may not be an integer in molecules with resonance or nonclassical bonding. The delocalized molecular orbitals of benzene contain 6 pi electrons over six carbons, thereby generating half a pi bond along with the sigma bond for each pair of carbon atoms, yielding a computed bond number of 1.5. Furthermore, bond numbers of 1.1, for example, can appear in complex settings and allude to bond strength in comparison to bonds of order 1.
CO Bond Order Explanation
The bond order in a covalent molecule represents the number of chemical bonds between two atoms. It is a measure of the strength and stability of a covalent bond. Bond order can be used to predict the relative bond lengths and bond energies in a molecule.
In the case of carbon monoxide (CO), there is a triple bond between the carbon (C) and oxygen (O) atoms. This means that there are three pairs of electrons shared between the carbon and oxygen atoms. Each pair of shared electrons constitutes a bond, so the bond order in carbon monoxide (CO) is 3.
The bond order is determined by counting the number of electron pairs that are shared between the two atoms in a covalent bond. Single bonds have a bond order of 1, double bonds have a bond order of 2, and triple bonds have a bond order of 3. In the case of CO, it’s a triple bond, so the bond order is 3.
Bond Order Formula
The bond order of a molecule is a measure of the number of chemical bonds between two atoms. It provides insight into the stability and properties of a molecule. The bond order can be calculated using the following formula:
Bond Order (BO) = (Number of Bonding Electrons – Number of Antibonding Electrons) / 2
In this formula:
- Number of Bonding Electrons: This is the total number of electrons in bonding molecular orbitals. These electrons are shared between the two atoms.
- Number of Antibonding Electrons: This is the total number of electrons in antibonding molecular orbitals. These electrons are not involved in bonding and tend to weaken the bond.
The division by 2 is necessary to ensure that the bond order is a whole number or a fraction. A bond order of 0.5 or less indicates a weak bond or the absence of a bond, while a bond order of 1 or more indicates a stable chemical bond.
For example, let’s calculate the bond order of a diatomic molecule like oxygen (O2):
Oxygen has 8 electrons each. In the molecular orbital diagram for O2, it has 2 electrons in a bonding molecular orbital (σ2s) and 4 electrons in antibonding molecular orbitals (σ2s and σ2p).
Using the formula, we get: Bond Order (O2) = (2 – 4) / 2 = (-2) / 2 = -1
So, the bond order of oxygen (O2) is -1, indicating a weak or antibonding interaction. This means that O2 is not a stable molecule in its ground state, and it exists as a diradical.
In contrast, for a molecule like nitrogen (N2), which has 3 bonding electrons and 2 antibonding electrons in its molecular orbitals:
Bond Order (N2) = (3 – 2) / 2 = 1/2 = 0.5
The bond order of nitrogen (N2) is 0.5, indicating a stable single bond between the nitrogen atoms.
Bond Order of CO +
The bond order of CO+ (carbon monoxide cation) can be calculated using molecular orbital theory. In molecular orbital theory, the bond order is determined by the difference in the number of bonding electrons and anti-bonding electrons divided by 2. Bond order = (number of bonding electrons – number of anti-bonding electrons) / 2.
Let’s analyze the molecular orbitals of CO+:
- Carbon (C) has 6 valence electrons, and oxygen (O) has 5 valence electrons. The positive charge on CO+ means it has lost one electron, so it has a total of 10 valence electrons.
- In molecular orbital theory, we construct molecular orbitals by combining atomic orbitals. The most important molecular orbital in CO+ is the sigma (σ) bonding orbital formed by the overlap of the 2p orbital of carbon and the 2p orbital of oxygen. This sigma bonding orbital is filled with 2 electrons.
- There is also a sigma star (σ*) anti-bonding orbital formed in the same manner, which is empty because there are no electrons in it.
Now, let’s calculate the bond order:
Bond order = (number of bonding electrons – number of anti-bonding electrons) / 2 Bond order = (2 – 0) / 2 Bond order = 2 / 2 Bond order = 1
So, the bond order of CO+ is 1. This indicates a single bond between carbon and oxygen in CO+.
CO Structure
Carbon monoxide (CO) is a chemical compound composed of one carbon (C) atom bonded to one oxygen (O) atom. The molecular structure of carbon monoxide is linear, with a triple bond between the carbon and oxygen atoms. The triple bond consists of two sigma (σ) bonds and one pi (π) bond, resulting in a very strong bond between the two atoms. The molecular formula for carbon monoxide is CO.
Here’s a simple representation of the carbon monoxide molecule:
O|
C
In this representation, the single lines (|) represent the sigma bonds, and the small pi symbol (π) can represent the pi bond.
Bond Order: Molecular Orbital Theory
Bond order is defined as half the difference between the number of bonding electrons and the number of antibonding electrons in molecular orbital theory, as seen in the equation below. For bonds around their equilibrium lengths, this generally but not always produces identical results, but it does not work for stretched bonds. Bond order is also utilised extensively in valence bond theory as a measure of bond strength.
The higher the bond order, the more powerful the bond. Half-bond orders might be stable.
Different Theories and Definitions for Bond Order
- For planar molecules with delocalized bonding, Molecular Orbital theory provides another method for establishing bond ordering based on MO coefficients. Bonding is divided into two systems in the theory: a sigma framework and a pi system. Charles Coulson used the orbital coefficients of the Hückel MOs to define the -bond order between atoms r and s derived from Hückel theory:
- In molecular dynamics and bond order potentials, the bond order notion is applied. The bond order’s magnitude is proportional to the bond length. The bond order between atoms I and j is experimentally stated as follows, according to Linus Pauling in 1947: where d1 is the single bond length, dij is the experimentally observed bond length, and b is a constant that varies with the atoms.
- Other formulations have been given for more complex types of MO theory with bigger basis sets For a long time, a conventional quantum mechanical definition for bond order has been contested. In 2017, a method for calculating bond ordering from quantum chemistry simulations was published.
Related Articles:
- What Is The Valency Of Nitrogen?
- Fathometer Is Used To Measure Ocean Depth
- Uidai.Gov.In My Aadhar- New Registration, Update, Download
- Who Wrote Mahabharata?- In Sanskrit, English, Hindi, Tamil, Telugu
What does a 1.5 bond order mean?
Because a resonance hybrid is formed by the mixing of two or more potential Lewis structures for the molecule or ion, a bond order of 1.5 indicates that there are resonance structures. A number like 1.5 might be obtained by averaging the bonds in each structure.
How do we find bond order?
Bond order is defined as half the difference between the number of bonding electrons and the number of antibonding electrons in molecular orbital theory. For bonds around their equilibrium lengths, this generally but not always produces identical results, but it does not work for stretched bonds. Bond order is also utilised extensively in valence bond theory as a measure of bond strength.
What does a 2.5 bond order mean?
Similarly, a bond order of 3 means that bonding orbitals have 6 more electrons than antibonding orbitals, resulting in a triple bond between the atoms. A bond order of 1.5 is more stable than a bond order of 1, while a bond order of 2.5 is more stable than a bond order of 2.
What is bond order used for?
The amount of covalent bonds that occur between two atoms is referred to as bond order. It’s also used to assess a bond’s stability, with a higher bond order indicating a more stable bond. This is due to increased electron attraction, which causes the atoms in the molecule to be bound together more securely.



![s_{ij} = \exp{\left[ \frac{d_{1} - d_{ij}}{b} \right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1f4eeab764914ea48d15e922d721018e428c4d3f) where d1 is the single bond length, dij is the experimentally observed bond length, and b is a constant that varies with the atoms.
where d1 is the single bond length, dij is the experimentally observed bond length, and b is a constant that varies with the atoms.






 UP NEET Counselling 2025 Round 5 Merit L...
UP NEET Counselling 2025 Round 5 Merit L...
 RBSE Class 10 Model Paper 2026 Out, Down...
RBSE Class 10 Model Paper 2026 Out, Down...
 RBSE Class 12 Model Paper 2026 for Arts,...
RBSE Class 12 Model Paper 2026 for Arts,...














