The Cannizzaro reaction is a chemical reaction named after Stanislao Cannizzaro in which two molecules of a non-enolizable aldehyde are disproportionated by a base to generate a carboxylic acid and a primary alcohol.
Cannizzaro Reaction
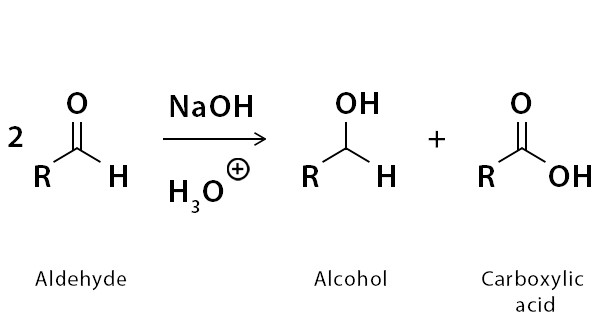
The Cannizzaro reaction is a chemical reaction that involves the disproportionation of an aldehyde into a carboxylic acid and an alcohol in the presence of a strong base. This reaction was discovered by the Italian chemist Stanislao Cannizzaro in 1853 and is an important transformation in organic chemistry.
The general equation for the Cannizzaro reaction is as follows:
2 RCHO + OH- → RCOOH + RCH2OH
In this reaction, two molecules of the same aldehyde (RCHO) react with a hydroxide ion (OH-) as a base catalyst. One aldehyde molecule is reduced to an alcohol (RCH2OH), while the other aldehyde molecule is oxidized to a carboxylic acid (RCOOH). The net effect is the disproportionation of the aldehyde into its corresponding carboxylic acid and alcohol.
Cannizzaro Reaction Definition
The Cannizzaaro Reaction is an aldehyde-caustic alkali reaction in which one molecule of aldehyde is reduced to the corresponding alcohol and another is oxidised to the corresponding acid’s salt. Cannizzaro first achieved this transformation in 1853, when he used potash to extract benzyl alcohol and potassium benzoate from benzaldehyde (potassium carbonate). More commonly, sodium hydroxide or potassium hydroxide would be used in the process, yielding the sodium or potassium carboxylate salt of the carboxylic-acid product:
| 2 C6H5CHO + KOH → C6H5CH2OH + C6H5COOK |
What is Cannizzaro Reaction for Class 12
The Cannizzaro reaction is typically carried out under basic conditions using strong bases like sodium hydroxide (NaOH) or potassium hydroxide (KOH). The mechanism involves the nucleophilic attack of hydroxide ions on the carbonyl carbon of one aldehyde molecule, leading to the formation of an alkoxide ion. This ion then reacts with another aldehyde molecule to form the carboxylic acid and alcohol products.
It’s important to note that not all aldehydes undergo the Cannizzaro reaction. Aldehydes that lack a hydrogen atom on the carbonyl carbon (i.e., hindered or sterically bulky aldehydes) usually do not undergo this reaction efficiently.
The Cannizzaro reaction is a significant synthetic tool in organic chemistry, allowing the conversion of aldehydes into their corresponding carboxylic acids and alcohols simultaneously. It also played a role in the historical development of understanding reaction mechanisms and the importance of electron transfer in chemical reactions.
Cannizzaro Reaction Mechanism Class 12
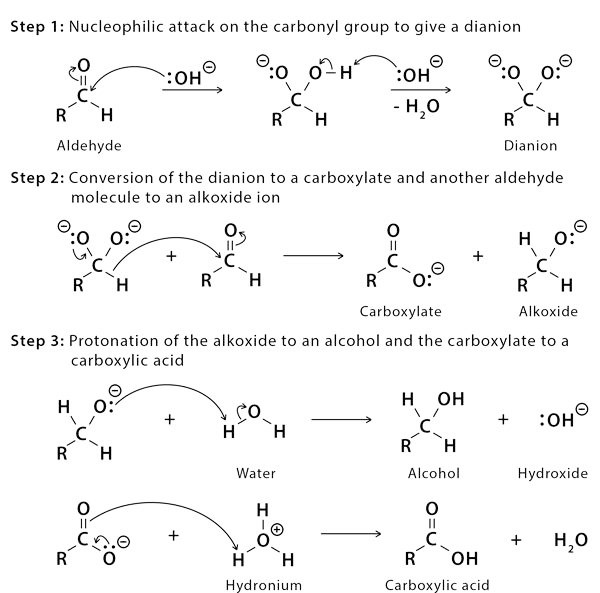
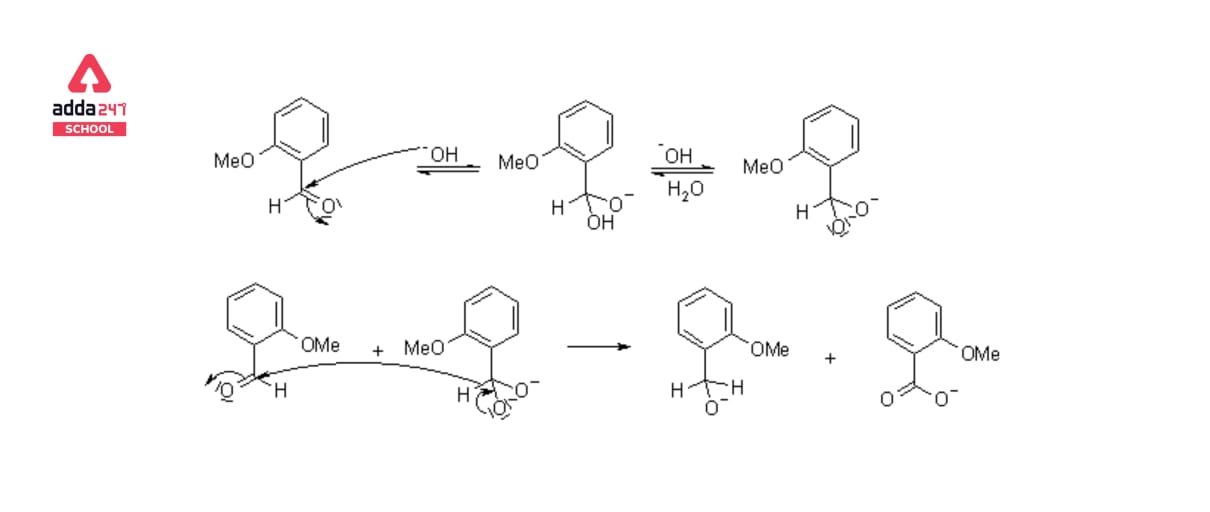
The Cannizzaro Reaction Mechanism explains how two molecules of an aldehyde can be converted into one molecule of alcohol and one molecule of carboxylic acid. Stanislao Cannizzaro succeeded in extracting benzyl alcohol and potassium benzoate from benzaldehyde in 1853. A nucleophilic acyl substitution on an aldehyde is used to carry out the reaction, with the leaving group attacking another aldehyde. The attack of hydroxide on a carbonyl produces a tetrahedral intermediate. When this tetrahedral intermediate collapses, the carbonyl is reformated and a hydride is transferred, which attacks another colony.
Acid and alkoxide ions are now exchanging a proton. When a high-concentration base is added, the aldehyde forms an anion with a charge of two. A hydride ion is then transferred to a second aldehyde molecule, resulting in carboxylate and alkoxide ions. For the reaction, the alkoxide ion additionally receives a proton from the solvent.
Mechanism of Cannizzaro Reaction Step by Step
The carbonyl group of the supplied aldehyde is attacked by a nucleophile, such as a hydroxide ion, resulting in a disproportionation reaction and the formation of an anion with two negative charges. This intermediate can now be used as a hydride reducing agent. The intermediate releases a hydride anion due to its unstable nature.
Another aldehyde molecule is attacked by this hydride anion. The aldehyde is now transformed into an alkoxide anion, and the doubly charged anion is changed into a carboxylate anion. Water provides a proton to the alkoxide anion in this penultimate phase, resulting in the final alcohol product. Because alkoxide is more basic than water, the reaction can continue. When acid workup is utilised, the carboxylate ion now produces the ultimate carboxylic acid product.
The equations steps of mechanism of Cannizzaro Reaction are given below:
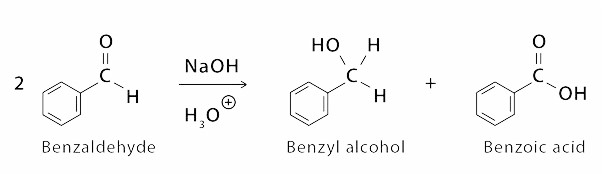
Cannizzaro Reaction Examples for Class 12
First Example is of formation of Benzoic Acid from Bezaldehyde
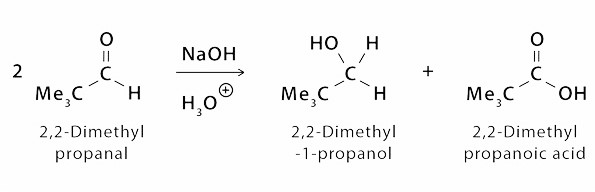
Another Example is of formation of 2,2 – dimethyl Propanoic Acid from 2,2 – di methyl Propanol
Cannizzaro Reaction Example for Class 12 Exams
The Cannizzaro reaction is a redox reaction involving the disproportionation of an aldehyde into a carboxylic acid and an alcohol in the presence of a strong base. This reaction is named after its discoverer, Stanislao Cannizzaro. Here are a couple of examples of the Cannizzaro reaction:
Example 1: Formaldehyde
Reaction:
HCHO + OH- → HCOO- + CH3OH
Formaldehyde → Formate ion + Methanol
Example 2: Benzaldehyde
Reaction:
C6H5CHO + 2 OH- → C6H5COO- + C6H5CH2OH
Benzaldehyde → Benzoate ion + Benzyl alcohol
In these examples, the aldehydes (formaldehyde and benzaldehyde) are converted into their corresponding carboxylic acids (formate ion and benzoate ion) along with the formation of alcohols (methanol and benzyl alcohol). The reaction requires a strong base, typically sodium hydroxide (NaOH) or potassium hydroxide (KOH), to deprotonate the aldehyde and initiate the reaction.
Keep in mind that the Cannizzaro reaction is most commonly observed in aldehydes that do not have a hydrogen atom in the alpha position (i.e., no hydrogen attached to the carbon next to the carbonyl group). Aldehydes with an alpha hydrogen typically undergo aldol condensation instead of the Cannizzaro reaction under basic conditions.
Explain Cannizzaro Reaction with Suitable Example.
The Cannizzaro reaction was discovered by Stanislao Cannizzaro, and it is named after him. Cannizzaro’s chemical interests were in natural products and aromatic compound reactions. He discovered in 1853 that when benzaldehyde is treated with concentrated base, it produces both benzoic acid and benzyl alcohol, a phenomenon known as the Cannizzaro reaction. The Cannizzaro Reaction Mechanism explains how two molecules of an aldehyde can be converted into one molecule of alcohol and one molecule of carboxylic acid.








 CUET 2026 Free Batches Launched by CUET ...
CUET 2026 Free Batches Launched by CUET ...
 CBSE Date Sheet 2026 for Class 10 & ...
CBSE Date Sheet 2026 for Class 10 & ...
 CBSE Class 10 Date Sheet 2026, Check 10t...
CBSE Class 10 Date Sheet 2026, Check 10t...














