Carbonic acid (H₂CO₃) is a weak, unstable acid that forms when carbon dioxide (CO₂) dissolves in water (H₂O). Although it exists only briefly in its pure form, carbonic acid plays a crucial role in many biological and environmental processes. In nature, it regulates pH levels in blood, assisting in respiratory and metabolic functions. Carbonic acid also influences the global carbon cycle, impacting ocean acidity and thus marine life. Despite being a transient molecule, its dissociation into bicarbonate and hydrogen ions makes it vital for buffering systems, contributing significantly to acid-base balance in various ecosystems and organisms.
Carbonic Acid
With the chemical formula H2CO3, carbonic acid is an inorganic substance in chemistry. It is common in water as a diluted solution, but only at temperatures about 80 °C can the pure component, a colourless gas, be produced. Contrary to popular assumption, the molecule is quite stable at room temperature in the absence of water, when it quickly breaks down into water and carbon dioxide. The interconversion of carbon dioxide and carbonic acid is influenced by both the acidity of natural waters and the breathing cycle of mammals.
Aqueous solutions of carbon dioxide frequently go by the name “carbonic acid” in biochemistry and physiology; they are crucial components of the bicarbonate buffer system, which regulates acid-base balance.
What is Carbonic Acid
Carbonic acid (H2CO3) is a weak, inorganic acid that forms when carbon dioxide (CO2) dissolves in water (H2O). It is a diprotic acid, meaning it can release two hydrogen ions (H+) per molecule in two stages of dissociation. The chemical reaction for the formation of carbonic acid from carbon dioxide and water can be represented as follows:
CO2 + H2O ⇌ H2CO3
Carbonic acid is a critical component in the carbon cycle, playing a significant role in natural processes. For example, it is involved in the dissolution of limestone and other carbonate minerals, which can lead to the formation of caves and karst landscapes. In biological systems, carbonic acid is also important because it helps regulate the pH of bodily fluids, such as blood, by acting as a buffer.
Its role in natural processes, carbonic acid is used in various industries, including the food and beverage industry, where it can be found in carbonated drinks like soda, as well as in some chemical and laboratory applications.
Carbonic Acid Formula
The chemical formula for carbonic acid is H2CO3. It is a weak acid that forms when carbon dioxide (CO2) dissolves in water (H2O), and it can release hydrogen ions (H+) in an aqueous solution. The chemical structure of carbonic acid consists of two hydroxide groups (OH-) and one carbonyl group (C=O) attached to a central carbon atom (C), along with two hydrogen atoms (H) bonded to the carbon atom.
Carbonic Acid Preparations
One carbon-oxygen double bond and two carbon-oxygen single bonds make up the structure of carbonic acid, as can be seen from the example given above. One hydrogen atom is joined to each of the oxygen atoms involved in a single bond with the carbon.
In many temperate habitats, carbonic acid, which is created when CO2 dissolves and hydrolyzes in water, is the main naturally occurring leaching agent. Because it is weak and unstable, carbonic acid quickly separates into bicarbonate ions and hydrogen ions (H+) (HCO3–)
Carbonic Acid Properties
The gaseous form of carbonic acid, H2CO3, is very stable at room temperature. It breaks down to produce carbon dioxide and water when there is water present, which speeds up the breakdown process.
Pure solid carbon dioxide is irradiated with proton beams to make pure carbonic acid, or hydrogen chloride and potassium bicarbonate react at 100 K in methanol to produce pure carbonic acid.
- A hybrid clamped cell made of a Russian alloy and copper-beryllium was used to create a high-pressure deuterated form of carbonic acid, or D2CO3, which was then studied by neutron diffraction.
- Planar molecules combine to create dimers that are connected by pairs of hydrogen bonds.
- At 1.34 Å, the three C-O bonds are almost equally spaced apart. Distances between C-O and C=O that are more common are 1.43 Å and 1.23 Å, respectively.
- Delocalized bonding in the molecule’s core and exceptionally strong hydrogen bonds, as evidenced by the O—-O separation of 2.13, are both responsible for the extraordinary C-O bond lengths.
- The 136° O-H-O enforced by the 8-membered rings with double hydrogen bonds is one cause of the short O—-O separation.
- Strong intramolecular hydrogen bonds, such as those in dicarboxylic acid, which have O—-O lengths above 2.4 Å, are known to occur.
- As ambient pressure carbonic acid does not exhibit Bragg peaks in X-ray diffraction, it must be categorised as amorphous.
Chemical Properties of Carbonic Acid
Following are the Chemical Properties of Carbonic Acid:
- Being a weak acid, H2CO3 is unstable in the natural world.
- When there is water present, it partially dissociates, releasing H+ and HCO3- (bicarbonate) ions.
- As carbonic acid is a diprotic acid, it can produce both bicarbonates and carbonates as salts.
- Bicarbonate salts are produced when a base is added in modest amounts to H2CO3, whereas carbonate salts are produced when a base is added in large amounts.
Physical Properties of Carbonic Acid
Following are the Physical Properties of Carbonic Acid:
- Carbonic acid has a molar mass of 62.024 grammes per mole.
- It has a density of 1.668 grammes per cubic centimetre in its normal state.
- The pKa value of the substance H2CO3 is 6.35.
- The bicarbonate is the conjugate base that corresponds to carbonic acid.
- The most common form of this chemical is a solution. Nonetheless, it has been stated that NASA scientists have generated solid H2CO3 samples.
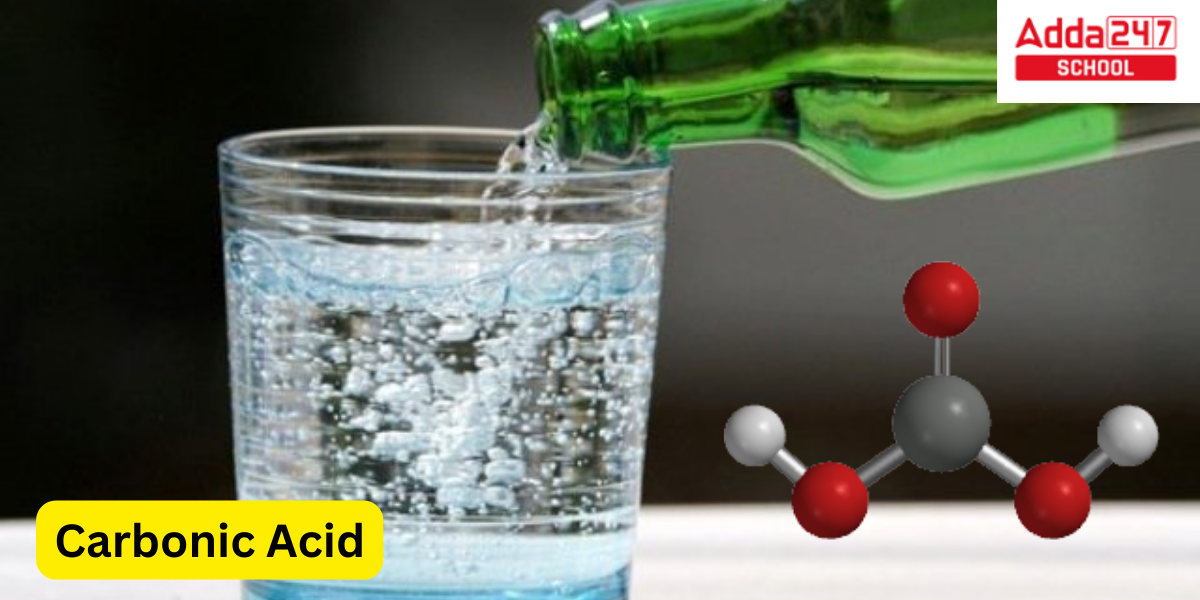
Carbonic Acid Structure
The structure of carbonic acid (H₂CO₃) is formed by bonding a carbon atom to two hydroxyl (–OH) groups and a double-bonded oxygen (C=O) atom. The structure can be described as follows:
Central Carbon (C): The central carbon atom is sp² hybridized.
Oxygen Atoms: There are three oxygen atoms attached to the carbon.
One oxygen is double-bonded (C=O).
The other two oxygens are single-bonded to the carbon and attached to hydrogen atoms (–OH).
The molecular geometry around the carbon atom is trigonal planar due to the three regions of electron density.
Here’s a simple representation of the carbonic acid structure:
H
|
H–O–C=O
|
O
|
H
In aqueous solution, carbonic acid readily dissociates into bicarbonate (HCO₃⁻) and hydrogen (H⁺) ions, making it an important compound in acid-base chemistry.
Carbonic Acid Uses
Following are the uses of Carbonic Acid:
- Carbonic acid is used in the production of sparkling wine, carbonated water, and other aerated beverages.
- The precipitation of several ammonium compounds, including ammonium persulfate, uses H2CO3.
- It facilitates the removal of carbon dioxide from the body.
- H2CO3 protonates a number of bases containing nitrogen in blood serum.
- Treatment for ringworm and other dermatitides involves applying carbonic acid to the affected region.
- Contact lens cleaning solutions that contain this substance work quite well.
- When necessary, it can be taken orally to cause vomiting (such as in drug overdose cases).
Carbonic Acid Uses in Real Life
Carbonic acid (H₂CO₃) plays a subtle yet important role in various real-life applications, often due to its weak acidic nature and role in maintaining balance in aqueous systems. Here are some of its practical uses:
Beverage Industry: Carbonic acid forms naturally when carbon dioxide (CO₂) is dissolved in water, creating the mild acidity in carbonated drinks, sparkling water, and sodas. This acid enhances flavor and preserves the drink by inhibiting microbial growth.
Biological Systems: In the human body, carbonic acid is crucial for maintaining blood pH balance. It forms when CO₂ dissolves in blood and helps regulate acid-base balance, primarily through its role in the bicarbonate buffer system.
Environmental Role: In nature, carbonic acid forms in rainwater as it absorbs atmospheric CO₂. This mildly acidic rainwater plays a role in weathering rocks, particularly limestone, and helps form soil over time. It also plays a role in forming caves and karst landscapes.
Aquarium and Fish Tank Maintenance: In aquariums, carbonic acid helps manage pH levels. Adding CO₂ to the water, where it forms carbonic acid, helps stabilize pH, benefiting fish and aquatic plants that require specific acidity levels.
Agricultural Applications: Carbonic acid helps release nutrients in the soil, especially in calcareous soils, by reacting with minerals. It assists in soil fertility and nutrient absorption by plants.
Industrial Cleaning: Due to its mild acidity, carbonic acid is sometimes used as a safer option for cleaning mineral deposits on surfaces, particularly in plumbing, where stronger acids might cause corrosion.
Though it’s a weak acid, carbonic acid’s buffering and dissolving abilities make it valuable across various fields, from health to environmental and industrial applications.
Carbonic Acid Importance
Importance of Carbonic Acid in Blood: It is well known that the bicarbonate ion functions as an intermediary in the process of respiratory gas exchange, which transports carbon dioxide from the human body. Carbon dioxide’s hydration processes go very slowly, especially when a sufficient catalyst is not present. Nevertheless, the carbonic anhydrases enzyme family is found in red blood cells, which speeds up the reaction.
The enzymes known as carbonic anhydrases catalyse the transformation of water and carbon dioxide into the dissociated ions of carbonic acid. This results in the formation of bicarbonate anions, which dissolve in the blood plasma. In the lungs, the catalysed reaction is reversed, creating CO2, which is subsequently breathed.
Importance of Carbonic Acid in the Ocean: The pH of the ocean’s water is thought to have changed by about -0.1 due to the oceans’ absorption of the extra carbon dioxide in the atmosphere, which is mostly the result of human activity. Ocean water and the absorbed carbon dioxide combine to make H2CO3. Ocean acidification is the term generally used to describe this process.
Related Article:
Ammonia Formula, Structure, Symbol
Slaked Lime Formula and Chemical Name, Uses
Plaster of Paris Formula, Properties and Uses








 CBSE Admit Card 2026 for Private & R...
CBSE Admit Card 2026 for Private & R...
 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...














