Table of Contents
CBSE Class 10 Science Answer Key 2025: The Central Board of Secondary Education successfully administered the Class 10 Science Examination of 2025 session. Our subject matter experts have published the CBSE Class 10 Science Answer Key 2025 pdf for each set listed below. In addition, the CBSE Class 10 Science question paper 2025 PDF has been included for reference, set by set. Students who will take the CBSE Science exam in 2026 must review the questions and answers for better preparation.
CBSE Class 10 Science Question Paper 2025
The Class 10 Science Exam 2025 was administered in pen and paper format. The CBSE Class 10 Science Answer Key 2025 included both objective and subjective types of questions. CBSE Class 10 Science Question Paper 2025 has a total of 80 marks. Students are allowed three hours to finish the board paper. An additional 15 minutes were allowed to read the CBSE 10th Science Question Paper 2025.
CBSE Class 10 Science Question Paper 2025 PDF
Students can download the CBSE Class 10 Science Question Paper 2025 PDF here. The Class 10th science paper consisted of Physics, Chemistry, and Biology. The CBSE Class 10th Science Board question paper contained both objective and subjective sorts of questions.
Students can download the CBSE Class 10 Science Question Paper 2025 PDF using the links provided below. CBSE Board Science Question Papers are essential resources since they allow students to analyze their test performance after the exam.
| CBSE Class 10 Science Paper 2025 PDFs | |
| Paper Set No | Download the Science Question Paper |
| Set 1 | Click Here |
| Set 2 | Click Here |
| Set 3 | Click Here |
CBSE Class 10 Science Answer Key 2025 OUT
Our educators published the set-by-set CBSE Class 10 Science Answer Key 2025 PDF on February 20, following the successful conclusion of the class 10th science board exam. This also provides an outline of papers to students preparing to take the class 10th exams in 2026. The direct links to download the CBSE Class 10 Science Answer Key 2025 sets 1, 2, and 3 PDF are provided here for free download.
| CBSE Class 10 Science Answer Key 2025 PDFs | |
| Paper No | Download the Full answer key science class 10 2025 |
| Set 1 | Download PDF |
| Set 2 | To be added soon |
| Set 3 | Download PDF |
Class 10 Science Answer Key 2025 – Highlights
Students can see all the questions from the exam and also obtain the CBSE Class 10 Science Answer Key 2025 in this section.
CBSE Class 10 Science Questions with Answers
Select and write the most appropriate option out of the four options given for each of the questions no. 1 to 20. There is no negative marking for incorrect response. 20×1=20

- In which of the following situations a chemical reaction does not occur?
Answer -Melting of Glaciers
- “In order to prepare dry hydrogen chloride gas in humid atmosphere the gas produced is passed through a guard tube (drying tube) which contain?”
Answer – Calcium Chloride
- The property by virtue of which a solid material can be drawn into thin wires is called Ductility
Select from the following a hydrocarbon having one C-C bond and one C— bond:
Answer –Cyclohexane
- The essential element taken up from the soil by the plants to synthesize proteins is:
Answer –Nitrogen
- “Select true statements about lymph from the following:
a. Lymph vessels carry ymph through the body and finally open into larger arteries
b. Lymph contains some amount of plasma, proteins and blood cells.
c. Lymph contains some amount of plasma, proteins and red blood cells.
d. Lymph vessels carry lymph through the body and finally open into larger veins.”
Answer –C and D
- Plants like Rose and Banana have lost the capacity to produce___
Answer – Seeds
In a bisexual flower the male gametes are present in the_____________
Answer –Anther
- “When a pure tall-tea plant i crossed with a pure dwarf pea plant, the percentage of tall pea plants in F 1 and F 2 generation pea plants will be respectively:”
Answer – 100%; 75%
- To get an image of magnification -1 on a screen using a length of focal length 20 cm, the object distance must be
Answer – 80 cm
- An optical device ‘X’ is placed obliquely in the path of a narrow parallel beam of light. If the emergent beam gets displaced laterally, the device ‘X’ is:
Answer –Convex lens
- A piece of wire of resistance ‘R’ is cut length-wise into three identical parts. These parts are then connected in parallel. If the equivalent resistance of this combination is R’, then the value of R/R’ is:”
Answer –1/9 - An electric bulb is rated 220V: 11W. The resistance of its filament when it glows with a power supply of 220 V is:
Answer –4400 Ω - “The minimum number of identical bulbs of rating 4V; 6W, that can work safely with desired
brightness, when connected in a series with a 240 V mains supply is:”
Answer –60 - “In the food chain given below. Select the most efficient food chain in terms of energy:
Grass → Grass hopper →Frog →Snake Plants → Deer → Lion
Plants → Man
Phytoplankton → Zooplankton →Small Fish →Big Fish”
Answer –“Grass → Grass
hopper →Frog →Snake” - Which one of the following gets biomagnified at different levels in a food chain?
Answer –DDT - “Assertion (A): In large animals, oxygen can reach different parts of the animal’s body easily.
Reason (R): Respiratory pigments take up oxygen from the air and carry it to body tissues.”
Answer –Both (A) and (R) are true and (R) is the explanation of (A). - Assertion (A): Concentrated nitric acid is diluted by adding water slowly to acid with constant stirring. Reason (R): Concentrated nitric acid is easily soluble in water.
Answer –Assertion (A) is true, but Reason (R) is false
- “Assertion (A): In reptiles, the temperature at which the fertilized eggs are kept decides the sex of the offspring
Reason (R): Sex is not genetically determined in some animals.”
Answer –“Both Assertion (A) and Reason (R) are true and Reason (R) is the
correct explanation of assertion” - “Assertion (A): When ciliary muscles contract, eye lens becomes thin.
Reason (R): Ciliary Muscles control the power of the eye lens.”
Answer –Assertion (A) is false but Reason (R) is true - The main observations while performing the
experiment of burning magnesium ribbon in air are:
(i) Magnesium ribbon burns with a dazzling white flame.
(ii) A white powder is formed
(iii) Magnesium ribbon vapourises
(iv) Aqueous solution of the white powder turns blue litmus to red”
Answer – i and ii - “A metal M, displaces iron from an aqueous solution of ferrous sulfate but fails to do so in case
of aqueous solution of aluminum suphate. The metal M is”
Answer – Zinc - “A common feature observed in the crystals of
washing soda, copper sulfate, gypsum, and ferrous sulphate is that all”
have fixed number of molecules of water of crystallisation in one formula unit of these salts
“A metal ‘X’ on treatment with sodium hydroxide
liberates a gas ‘G’. It also liberates the same gas, ‘G’ on treatment with dilute sulphuric acid.”
Answer – Zinc and hydrogen - “The values of a,b,c and d in the following balanced chemical equation are respectively:
a Pb (NO 3 ) 2 → bPbO + cNO 2 + dO 2”
Answer – 1,1,2,1
- During electrolytic refining of copper, the anode, the cathode, and the electrolyte used respectively are ”
Answer – Impure copper, pure copper, acidified copper sulphate solution” - If we make carbon skeleton with four carbon atoms, the two different possible skeletons will be
Answer – C-C-C-C, C-C / / C - Listed below are the steps of nutrition in Ameoba. Select the correct sequence of the steps.
Diffusion of simple nutrients into cytoplasm
(i) Food vacuole formation
(ii) Formation of finger-like temporary extensions of cell surface
(iii) Complex substances broken to simpler ones
(iv) Undigested material thrown out of the cell surface”
Answer – iii, ii, iv, i, v - “Which among the following is not a neural action
controlled by the part of human brain labeled ‘X’ in the figure above?”
Answer –Hunger - The modes of reproduction in Spirogyra and Planaria respectively are
Answer – Fragmentation and regeneration
Q. Consider the following chemical equation :
p Al + q H2O→r Al2O3 + 5 H2
To balance this chemical equation, the values of ‘p’, ‘q’, ‘r’ and ‘s’ must be respectively:
(A) 3, 2, 2, 1
(B) 2, 3, 3, 1
(C) 2, 3, 1, 3
(D) 3, 1, 2, 2
Q. Which of the given option represents a family of salts?
(A) NaCl, Na2SO4, CaSO4
(B) K2SO4, Na2SO4, CaSO4
(C) NaNO3, CaCO3, Na2CO3
(D) MgSO4, CuSO4, MgCl2
Q. The most common method of extraction of metals from their oxide ores is:
(A) Reduction with carbon
(B) Reduction with hydrogen
(C) Reduction with aluminium
(D) Electrolytic reduction
4. Given below are the structures of some hydrocarbons. Select the two structures which are related to each other from the given options:
(i) and (iv)(B)
(ii) and (iv)(C)
(ii) and (iii)
(D) (i) and (iii)
5. Choose the incorrect statement about the common reaction used in hydrogenation of vegetable oils.
(A) It is an addition reaction.
(B) It takes place in the presence of nickel or palladium catalyst.
(C) The product contains only single bonds between carbon atoms.
(D) It is an addition reaction which occurs in the presence of an acid catalyst.
Class 10 Science Board Paper 2025 Exam Analysis
- The Science board paper ended at 1.30 pm. We have updated the student’s and teachers’ reactions on this page.
- The students and teachers said that the paper was easy.
- 70 to 80 % questions were common.
- the teachers said that the Science question paper was moderate.
| Science Exam Analysis 2025 | |
|
Overall Difficulty
|
Easy to Moderate
|
|
Key Analysis
|
|
|
Student Feedback
|
|
|
Comparison with Sample Paper
|
Easier than the Sample Paper
|
|
Good Attempts
|
75 out of 80 Marks
|
|
NCERT Relevance
|
NCERT was sufficient for scoring well in the exam
|
Check the complete Science Exam Analysis 2025 here –
CBSE Board 10th Science Questions 2025
The CBSE Board previously released the CBSE Class 10th Science Sample Question Paper 2025 on its official website to assist students. These important sample questions help in completing their preparations with the best practice resources. The board releases all of the Class 10th Science Question Papers 2025 to let students become familiar with the exam methodology, exam pattern, and marking scheme used in the final exam. Students can also check the list of important and expected questions for this year’s board exam. Make sure to go through all of them to score excellent marks.
Light Refraction and Reflection
Q1. List four characteristics of the images formed by plane mirrors. (Delhi 2015, AI2011)
Answer:
Characteristics of the image formed by a plane mirror are
(i) image distance is same as that of object distance
(ii) image formed is virtual and erect
(iii) image formed is of the same size as that of the object
(iv) image formed is laterally inverted (left appears right and right appears left).
Q2. An object is placed at a distance of 12 cm in front of a concave mirror of radius of curvature 30 cm. List four characteristics of the image formed by the mirror. (Delhi 2017)
Answer:
Radius of curvature (R) = 30 cm, object distance is 12 cm in front of the mirror. Thus we can say that object is placed between focus and pole. Four characteristics of the image formed by die given concave mirror when object is placed between pole and focus are:
(i) Virtual
(ii) Erect
(iii) Enlarged
(iv) Image is formed behind the mirror
Chemical Reaction and Equations
- (a) State the law that is followed by balancing a chemical equation.
(b) Balance the following chemical equation: Na + H3O → NaOH + H2 (Board Exam, 2013)
Answer:
(a) Law of conservation of mass is followed for balancing a chemical equation which states that mass can neither be created nor destroyed in a chemical reaction. That is, the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants in a balanced equation.
(b) 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) - Calcium oxide reacts vigorously with water to produce slaked lime.
CaO(s) + H2O(l) → Ca(OH)2(aq)
This reaction can be classified as
(A) Combination reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
Which of the following is a correct option? (2020)
(a) (A) and (C)
(b) (C) and (D)
(c) (A), (C) and (D)
(d) (A) and (B)
Answer:
(d) The reaction between CaO and H2O to form Ca(OH)2 is an exothermic combination reaction. - State the type of chemical reactions, represented by the following equations : (Board Term I, 2014)
(a) A + BC → AC + B
(b) A + B → C
(c) PQ + RS → PS + RQ
(d) A2O3 + 2B → B2O3 + 2A
Answer:
(a) Displacement reaction.
(b) Combination reaction.
(c) Double displacement reaction.
(d) Displacement reaction or redox reaction.
Heredity and Evolution
Q 1. “Only variations that confer an advantage to an individual organism will survive in a population.” Justify this statement. (Foreign 2011)
Answer:
Variations are the structural, functional or behavioural changes from the normal characters developed in the living organisms. Inheritable variations participate in evolution. According to Darwin, natural selection sorts out individuals with favourable variations. Such organism will survive, reproduce more and thus, will leave more progenies. Hence, useful variations get established in nature.
Q 2. What is a gene? (AI 2014)
Answer:
A gene is a unit of DNA on a chromosome which governs the synthesis of particular protein that controls specific characteristics (or traits) of an organism.
Q 3. Write a difference between inherited traits and acquired traits giving one example of each. (Delhi 2013C)
Answer:
A trait (or characteristic) of an organism which is ‘not inherited’ but develops in response to the environment is called an acquired trait. For example, if a group of mice are normally bred, all their progeny will have tails. Now, if the tails of these mice are cut by surgery in each generation, tail-less mice will not be produced. This is so because removal of tail is an acquired character.
Q 4. (a) Why did Mendel carry out an experiment to study inheritance of two traits in garden pea?
(b) What were his findings with respect to inheritance of traits in F1 and F2 generation?
(c) State the ratio obtained in the F2 generation in the above mentioned experiment. (2020)
Answer:
(a) Mendel carried out crosses with two traits to see the interaction and basis of inheritance between them. In a dihybrid cross given by Mendel, it was observed that when two pairs of characters were considered each trait expressed independent of the other.
b) For example, a cross between round yellow and wrinkled green parents.
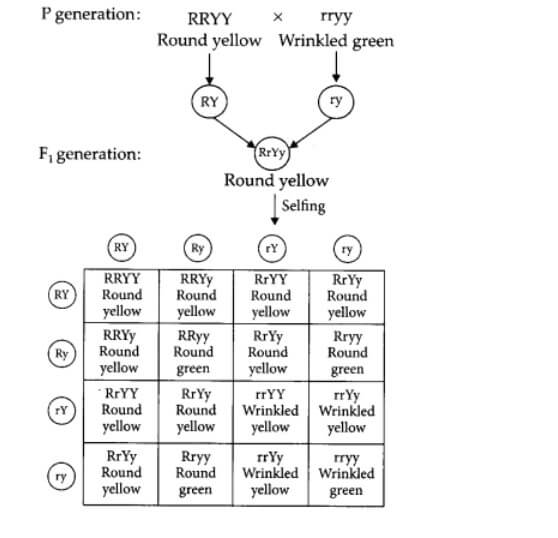 In F1 generation, all plants are with round yellow seeds. But in F2 generation, we find all types of plants : Round yellow, Round green, Wrinkled yellow, Wrinkled green.
In F1 generation, all plants are with round yellow seeds. But in F2 generation, we find all types of plants : Round yellow, Round green, Wrinkled yellow, Wrinkled green.
F2 generation ratio : Round-yellow = 9 : Round- green = 3 : Colour of stem in F1 progeny Wrinkled- yellow = 3 : Wrinkled-green = 1
Carbon and Its Compound
Q 1. Give reasons for the following:
(i) Element carbon forms compounds mainly by covalent bonding.
(ii) Diamond has high melting point.
(iii) Graphite is a good conductor of electricity. (3/5, Foreign 2011)
Answer:
(i) As carbon has four valence electrons and it can neither loose nor gain lour electrons thus, it attains noble gas configuration only by sharing of electrons. I bus, it forms covalent compounds.
(ii) In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure. This makes diamond the hardest known substance. Thus, it has high melting point.
(iii) In graphite, each carbon atom is bonded to three other carbon atoms by covalent bonds in the same plane giving a hexagonal array. Thus, only three valence electrons are used for bond formation and hence, the fourth valence electron is free to move. As a result, graphite is a good conductor of electricity
Q 2. What are covalent bonds? Show their formation with the help of electron dot structure of methane. Why are covalent compounds generally poor conductors of electricity? (Delhi 2013C)Covalent bond jewelryMolecule model kits
Answer:
Covalent bonds are those bonds which are formed by sharing of the valence electrons between two atoms. Electron dot structure of methane is shown in the figure.
Carbon and its Compounds Chapter Wise Important Questions Class 10 Science Img 1
Covalent compounds are generally poor conductors ol electricity because they do not have tree electrons or ions.
Q 3. Name a cyclic unsaturated carbon compound. (2020)
Answer:
Benzene
Acid, Bases, Salts
- Q1. 2 mL of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test tube. When the content are warmed, a gas evolves which is bubbled through a soap solution before testing. Write the equation of the chemical reaction involved and the test to detect the gas. Name the gas which will be evolved when the same metal reacts with dilute solution of a strong acid.
Answer:
It is observed that active metals like zinc react with strong bases like NaOH, KOH etc. to liberate hydrogen gas and corresponding salt.
Acids Bases and Salts Class 10 Important Questions with Answers Science Chapter 2 Img 4
The evolution of gas is confirmed by the bubble formation in soap solution.
Test to detect H2 gas: When burning matchstick is kept on the mouth of this test tube, pop sound is heard which confirms the presence of H2 gas. When Zn metal reacts with dilute solution of strong acid, H2 gas is evolved.
Acids Bases and Salts Class 10 Important Questions with Answers Science Chapter 2 Img 5
- Q 2. To. a solution of sodium hydroxide in a test tube, two drops of phenolphthalein are added.
(i) State the colour change observed.
(ii) If dil HCl is added dropwise to the solution, what will be the colour change?
(iii) On adding few drops of NaOH solution to the above mixture the colour of the solution reappears. Why? (Board Term I, 2013)
Answer:
(i) On adding phenolphthalein to NaOH solution, the colour becomes pink.
(ii) On adding dilute HCl solution dropwise to the same test tube, the pink colour disappears and the solution again becomes colourless.
(iii) On again adding NaOH to the above mixture, pink colour reappears because the medium becomes basic again. - Q 3. (a) Write the chemical name and formula of marble.
(b) It has been found that marbles of Taj are getting corroded due to development of industrial areas around it. Explain this fact giving a chemical equation.
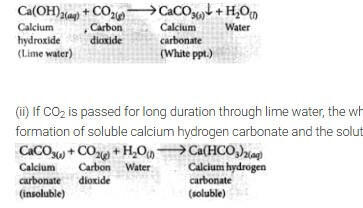
(c) (i) What happens when CO2 is passed through lime water?
(ii) What happens when CO2 is passed in excess through lime? (Board Term I, 2013)
Answer:
(a) The chemical formula of marble (lime stone) is CaCO3. Its chemical name is calcium carbonate.
(b) Taj Mahal, one of the seven wonders of the world situated at Agra, is continuously losing its luster day by day due to rapid industrialisation which causes acid rain.
The sulphuric acid present in the acid rain causes the marble (CaCO3) to be washed off as calcium sulphate (CaSO4), leading to the deterioration of such a splendid piece of architecture.
CaCO3(s) + H2SO4(aq) → CaSO4(aq) + H2Ol + CO2(g)
(c) Refer to answer 2.
Periodic Classification
Q1. Why did Mendeleev leave some gaps in the Periodic table?
Answer:
Mendeleev left some gaps in the periodic table for yet to be discovered elements. Mendeleev predicted the properties of these elements on the basic of their positions. For example, he predicted the properties of gallium (eka-aluminium) and germanium (eka-silicon) which were unknown at that time.
Q 2. An element ‘X’ is forming an acidic oxide. Its position in modern periodic table will be
(a) group 1 and period 3
(b) group 2 and period 3
(c) group 13 and period 3
(d) group 16 and period 3. (2020)
Answer:
(d) : As the element X forms an acidic oxide, hence ‘X is a non-metal. Hence, X is sulphur.
Q 3. List any two properties of the elements belonging to the first group of the Modern Periodic Tablet (AI 2014)
Answer:
Two properties of the elements belonging to the first group:
(i) As the elements belong to group 1, so they have one electron in their outermost shell hence, valency of these elements is one.
(ii) Alkali metals (group 1 elements) are electropositive in nature.
Life Process
Q 1. State the location and function of gastric glands. (Board Term I, 2014)
Answer:
Gastric glands are present in the wall of the stomach. They secrete gastric juices containing mucus, protein digesting enzymes pepsin, rennin and hydrochloric acid (HCl).
Q 2. (a) State the role played by the following in the process of digestion :
(i) Enzyme trypsin
(ii) Enzyme lipase-
(b) List two functions of finger-like projections present in the small intestine. (2020)
Answer:
(a) (i) Enzyme trypsin : This enzyme is produced by the pancreas in an inactive form called trypsinogen. Trypsin converts remaining proteins into peptones and the peptones into peptides and amino acids.
(ii) Enzyme lipase : It is secreted by pancreas and small intestine. Lipase converts fats into fatty acids and glycerol.
(b) Internally, the wall of the small intestine is provided with long finger-like projections called villi. Two functions of villi are :
(i) The villi greatly increase the absorptive surface area of the inner lining of small intestine.
(ii) The large surface area of small intestine helps in rapid absorption of digested food.
Q 3. (a) What is peristaltic movement?
(b) ‘Stomata remain closed in desert plants during daytime’. How do they do photosynthesis? (Board Term I, 2013)
Answer:
(a) The relaxation of gut muscles to move the partially digested food downwards throughout the alimentary canal is called peristaltic movement.
(b) In desert plants, stomata open at night and take in carbon dioxide (CO2). Stomata remain closed during daytime to prevent the loss of water by transpiration. They store the CO2 in their cells until the sun comes out so that they can carry on with photosynthesis during the daytime.
| Related Articles | |
| CBSE Class 10 English Answer Key 2025 | CBSE Date Sheet 2025 |
| CBSE Science Paper Leak 2025 Read Full News | CBSE Class 10 Hindi Answer Key 2025 with Answer Key |



 JEE Mains 2025 Session 2 Exam Live Updat...
JEE Mains 2025 Session 2 Exam Live Updat...
 CBSE Class 12 Home Science Answer Key 20...
CBSE Class 12 Home Science Answer Key 20...
 CBSE Class 12 History Answer Key 2025, G...
CBSE Class 12 History Answer Key 2025, G...










