Table of Contents
Chemical formula: Each constituent element in a compound is recognized by its chemical symbol, and the chemical formula shows the proportionate number of atoms. A chemical formula is the use of chemical element symbols, numbers, and, on occasion, other symbols such as parentheses, dashes, brackets, commas, and plus (+) and minus (-) signs to represent information about the chemical proportions of atoms that make up a specific chemical compound or molecule.
Chemical Formula
The chemical formula represents the chemical composition of a compound symbolically. Chemical formulas show the components that comprise the molecules of a chemical, as well as the ratio whereby their atoms join to produce those molecules. A molecule of water, for example, is made up of two hydrogen atoms and one oxygen atom, according to the molecular formula of water, H2O.
Acetone Formula, Structure, Name, Uses, Reactions in Chemistry
These proportions in empirical equations begin with a key element before assigning atom counts for the remaining components of the compound in connection with the key element. When applied to molecular compounds, these ratio values are all whole integers. Because all ethanol molecules contain two carbon atoms, six hydrogen atoms, and one oxygen atom, its empirical formula is C2H6O.
Chemical Formula Definition
A chemical formula identifies each element by its chemical symbol and indicates the proportionate number of atoms of each element. In chemical formulae, these proportions begin with a key element and then assign numbers of atoms of the other elements in the compound, by ratios to the key element.
Gold Formula in Chemistry, Check Chemical Formula of Gold
Chemical Formula Types
While the phrase ‘chemical formula’ normally refers to a compound’s molecular formula, the compositions of chemical compounds can be stated in a variety of ways, which are detailed below.
- Molecular Formula: The molecular formula gives information about the number of elements in a compound. The elements are denoted by their respective symbols (as in the periodic table) in molecular formulae, and the number of atoms of each element in the molecule is written in subscripts. The chemical formula for glucose, for example, is C6H12O6.
- Empirical Formula: The empirical formula of a chemical compound represents the elemental ratio of that substance. Typically, empirical equations are derived from the examination of experimental data. One carbon atom, three hydrogen atoms, and one oxygen atom make up the simplest ratio of the empirical formula for ethanol, which is CH3O.
- Structural formula: The atoms of a molecule are arranged in this type of chemical formula.
- Ball-and-stick model: This is a picture of a molecule in three dimensions. Molecule structures and their interactions can be depicted using ball-and-stick models.
Aluminium Fluoride Formula- Structure, Chemical Name, Uses
How Do I Write a Chemical Formula?
A chemical formula, as previously stated, is a symbolic statement that represents the number of atoms contained in a molecular material. We can tell what type of atom it is by its symbol, thus for Hydrogen, we’ll use H. The subscript linked to the symbol determines the number of atoms. Water has two hydrogen atoms and one oxygen atom in its chemical formula, H2O.
How to Write a Chemical Formula –
Step 1: First, you must determine the sort of bond.
If the prefixes are utilized, the bond is covalent. It is an ionic bond if there are no prefixes.
Step 2: Make a note of the symbol for the polyatomic ion or element.
Step 3: If the prefix was utilized, you must now add a subscript. To balance the charge, you’ll also need to include a subscript.
All Chemical Formula Tables for Class 11 and 12
The table below consists of various chemical formulas.
| Chemical Formula Table | ||
| Sl.No | Name of the Chemical Compound | Formula |
| 1 | Acetic acid formula | CH3COOH |
| 2 | Aluminium hydroxide formula | Al(OH)3 |
| 3 | Acetate formula | CH3COO- |
| 4 | Acetone formula | C3H6O |
| 5 | Aluminum acetate formula | C6H9AlO6 |
| 6 | Aluminium bromide formula | AlBr3 |
| 7 | Aluminum carbonate formula | Al2(CO3)3 |
| 8 | Aluminium chloride formula | AlCl3 |
| 9 | Aluminium fluoride formula | AlF3 |
| 10 | Aluminium formula | Al |
| 11 | Aluminium iodide formula | AlI3 |
| 12 | Aluminium oxide formula | Al2O3 |
| 13 | Aluminium phosphate formula | AlPO4 |
| 14 | Amino acid formula |
H2NCHRCOOH
|
| 15 | Ammonia formula | NH3 |
| 16 | Ammonium dichromate formula | Cr2H8N2O7 |
| 17 | Ammonium acetate formula | C2H3O2NH4 |
| 18 | Ammonium bicarbonate formula | NH4HCO3 |
| 19 | Ammonium bromide formula | NH4Br |
| 20 | Ammonium carbonate formula | (NH4)2CO3 |
| 21 | Ammonium chloride formula | NH4Cl |
| 22 | Ammonium hydroxide formula | NH4OH |
| 23 | Ammonium iodide formula | NH4I |
| 24 | Ammonium nitrate formula | NH4NO3 |
| 25 | Aluminium sulfide formula | Al2S3 |
| 26 | Ammonium nitrite formula | NH4NO2 |
| 27 | Ammonium oxide formula | (NH4)2O |
| 28 | Ammonium phosphate formula | (NH4)3PO4 |
| 29 | Ammonium sulfate formula | (NH4)2SO4 |
| 30 | Ammonium sulfide formula | (NH4)2S |
| 31 | Argon gas formula | Ar |
| 32 | Ascorbic acid formula | C6H8O6 |
| 33 | Barium acetate formula |
Ba(C2H3O2)2
|
| 34 | Barium bromide formula | BaBr2 |
| 35 | Barium chloride formula | BaCl2 |
| 36 | Barium fluoride formula | BaF2 |
| 37 | Barium hydroxide formula | Ba(OH)2 |
| 38 | Barium iodide formula | BaI2 |
| 39 | Barium nitrate formula | Ba(NO3)2 |
| 40 | Barium oxide formula | BaO |
| 41 | Barium phosphate formula | Ba3O8P2 |
| 42 | Barium sulfate formula | BaSO4 |
| 43 | Benzene formula | C6H6 |
| 44 | Benzoic acid formula | C7H6O2 |
| 45 | Bicarbonate formula | CHO3– |
| 46 | Bleach formula | NaClO |
| 47 | Boric acid formula | H3BO3 |
| 48 | Potassium Bromate formula | KBrO3 |
| 49 | Bromic acid formula | HBrO3 |
| 50 | Bromine formula | Br |
| 51 | Butane formula | C4H10 |
| 52 | Butanoic acid formula | C4H8O2 |
| 53 | Calcium acetate formula | C₄H₆CaO₄ |
| 54 | Calcium bromide formula | CaBr2 |
| 55 | Calcium carbonate formula | CaCO3 |
| 56 | Calcium hydride formula | CaH2 |
| 57 | Calcium hydroxide formula | Ca(OH)2 |
| 58 | Calcium iodide formula | CaI2 |
| 59 | Calcium nitrate formula | Ca(NO3)2 |
| 60 | Calcium oxide formula | CaO |
| 61 | Carbon monoxide formula | CO |
| 62 | Carbon tetrachloride formula | CCl4 |
| 63 | Carbonic acid formula | H2CO3 |
| 64 | Calcium phosphate formula | Ca3(PO4)2 |
| 65 | Carbonic acid formula | H2CO3 |
| 66 | Citric acid formula | C6H8O7 |
| 67 | Chlorate formula | ClO–3 |
| 68 | Chlorine formula | Cl |
| 69 | Chlorine gas formula | Cl2 |
| 70 | Chlorous acid formula | HClO2 |
| 71 | Chromate formula | CrO42- |
| 72 | Chromic acid formula | H2CrO4 |
| 73 | Citric acid formula | C6H8O7 |
| 74 | Copper ii carbonate formula | CuCO3 |
| 75 | Copper ii nitrate formula | Cu(NO3)2 |
| 76 | Cyanide formula | CN– |
| 77 | Dichromate formula | K2Cr2O7 |
| 78 | Dihydrogen monoxide formula | H2O |
| 79 | Dinitrogen monoxide formula | N2O |
| 80 | Dinitrogen pentoxide formula | N2O5 |
| 81 | Dinitrogen trioxide formula | N2O3 |
| 82 | Ethanol formula | C2H5OH |
| 83 | Iron oxide formula | Fe2O3 |
| 84 | Ethylene glycol formula | C2H6O2 |
| 85 | Fluorine gas formula | F2 |
| 86 | Aluminum bromide formula | AlBr3 |
| 87 | Aluminum sulfide formula | Al2S3 |
| 88 | Ammonium carbonate formula | (NH4)2CO3 |
| 89 | Ammonium nitrate formula | (NH4)(NO3) |
| 90 | Ammonium phosphate formula | (NH4)3PO4 |
| 91 | Barium chloride formula | BaCl2 |
| 92 | Barium sulfate formula | BaSO4 |
| 93 | Calcium nitrate formula | Ca(NO3)2 |
| 94 | Carbon monoxide formula | CO |
| 95 | Carbon tetrachloride formula | CCl4 |
| 96 | Carbonic acid formula | H2CO3 |
| 97 | Hydrofluoric acid formula | HF |
| 98 | Hydroiodic acid formula | HI |
| 99 | Hypochlorous acid formula | HClO |
| 100 | Lithium phosphate formula | Li3PO4 |
| 101 | Magnesium nitrate formula | MgNO3 |
| 102 | Magnesium phosphate formula | Mg3(PO4)2 |
| 103 | Nitrogen monoxide formula | NO |
| 104 | Nitrous acid formula | HNO2 |
| 105 | Potassium carbonate formula | K2CO3 |
| 106 | Potassium iodide formula | KI |
| 107 | Potassium nitrate formula | KNO3 |
| 108 | Potassium phosphate formula | KH2PO4 |
| 109 | Sodium carbonate formula | Na2CO3 |
| 110 | Sodium oxide formula | Na2O |
| 111 | Fructose chemical formula | C6H12O6 |
| 112 | Glycerol formula | C3H8O3 |
| 113 | Helium gas formula | He |
| 114 | Hexane formula | C6H14 |
| 115 | Hydrobromic acid formula | HBr |
| 116 | Hydrochloric acid formula | HCl |
| 117 | Hydrocyanic acid formula | HCN |
| 118 | Hydrofluoric acid formula | HF |
| 119 | Hydrogen carbonate formula | CHO3– |
| 120 | Hydrogen gas formula | H2 |
| 121 | Hydrogen peroxide formula | H2O2 |
| 122 | Hydrogen phosphate formula | H3PO4 |
| 123 | Hydrogen sulfate formula | HSO4– |
| 124 | Hydroiodic acid formula | HI |
| 125 | Hydrosulfuric acid formula | H2SO4 |
| 126 | Hydroxide ion formula | OH– |
| 127 | Hypobromous acid formula | HBrO |
| 128 | Hypochlorite formula | NaClO |
| 129 | Hypochlorous acid formula | HClO |
| 130 | Hypoiodous acid formula | HIO |
| 131 | Iodic acid formula | HIO3 |
| 132 | Iodide ion formula | I– |
| 133 | Iodine formula | I2 |
| 134 | Iron (iii) nitrate formula | Fe(NO3)3 |
| 135 | Iron (ii) oxide formula | FeO |
| 136 | Iron (iii) carbonate formula | Fe2(CO3)3 |
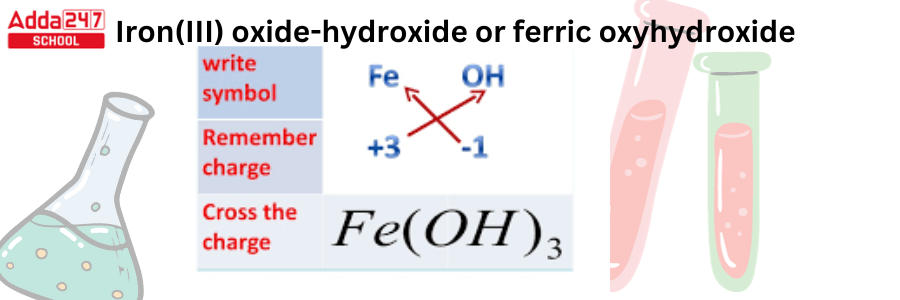
| 137 | Iron (iii) hydroxide formula | Fe(OH)3 |
| 138 | Iron (iii) oxide formula | Fe2O3 |
| 139 | Iron (iii) chloride formula | FeCl3 |
| 140 | Lactic acid formula | C3H6O3 |
| 141 | Lead acetate formula |
Pb(C2H3O2)2
|
| 142 | Lead (ii) acetate formula |
Pb(C2H3O2)2
|
| 143 | Lead iodide formula | PbI2 |
| 144 | Lead (iv) oxide formula | PbO2 |
| 145 | Lead nitrate formula | Pb(NO3)2 |
| 146 | Lithium bromide formula | LiBr |
| 147 | Lithium chloride formula | LiCl |
| 148 | Lithium hydroxide formula | LiOH |
| 149 | Lithium iodide formula | LiI |
| 150 | Lithium oxide formula | Li2O |
| 151 | Lithium phosphate formula | Li3PO4 |
| 152 | Magnesium acetate formula |
Mg(CH3COO)2
|
| 153 | Magnesium bicarbonate formula | C2H2MgO6 |
| 154 | Magnesium carbonate formula | MgCO3 |
| 155 | Magnesium chloride formula | MgCl2 |
| 156 | Magnesium hydroxide formula | Mg(OH)2 |
| 157 | Magnesium iodide formula | MgI2 |
| 158 | Magnesium nitrate formula | Mg(NO3)2 |
| 159 | Magnesium nitride formula | Mg3N2 |
| 160 | Magnesium carbonate formula | MgCO3 |
| 161 | Magnesium bromide formula | MgBr2 |
| 162 | Magnesium oxide formula | MgO |
| 163 | Magnesium phosphate formula | Mg3(PO4)2 |
| 164 | Magnesium sulfate formula | MgSO4 |
| 165 | Magnesium sulfide formula | MgS |
| 166 | Methane formula | CH4 |
| 167 | Methanol formula | CH3OH |
| 168 | Nickel acetate formula | Ni(C2H3O2)2 |
| 169 | Nickel nitrate formula | Ni(NO3)2 |
| 170 | Nitric acid formula | HNO3 |
| 171 | Nitride formula | N3– |
| 172 | Nitrite formula | NO2− |
| 173 | Nitrogen dioxide formula | NO2 |
| 174 | Nitrogen monoxide formula | NO |
| 175 | Nitrous acid formula | HNO2 |
| 176 | Oxalate formula | C2O42− |
| 177 | Oxalic acid formula | H2C2O4 |
| 178 | Oxygen formula | O2 |
| 179 | Ozone formula | O3 |
| 180 | Perbromic acid formula | HBrO4 |
| 181 | Potassium Permanganate formula | KMnO4 |
| 182 | Permanganate ion formula | MnO4– |
| 183 | Phosphate formula | PO43- |
| 184 | Sodium hydrogen phosphate formula | Na2HPO4 |
| 185 | Sodium formate formula | HCOONa |
| 186 | Phosphoric acid formula | H3PO4 |
| 187 | Phosphorus pentachloride formula | PCl5 |
| 188 | Phosphorus trichloride formula | PCl3 |
| 189 | Potassium acetate formula | CH3CO2K |
| 190 | Potassium bicarbonate formula | KHCO3 |
| 191 | Potassium carbonate formula | K2CO3 |
| 192 | Potassium chlorate formula | KClO3 |
| 193 | Potassium hydrogen phosphate formula | K2HPO4 |
| 194 | Potassium chloride formula | KCl |
| 195 | Potassium chromate formula | CrK2O4 |
| 196 | Potassium cyanide formula | KCN |
| 197 | Potassium dichromate formula | K2Cr2O7 |
| 198 | Potassium fluoride formula | KF |
| 199 | Potassium hydroxide formula | KOH |
| 200 | Potassium hypochlorite formula | KClO |
| 201 | Potassium iodide formula | KI |
| 201 | Potassium dihydrogen phosphate formula | KH2PO4 |
| 203 | Potassium nitrate formula | KNO3 |
| 204 | Potassium nitrite formula | KNO2 |
| 205 | Potassium oxide formula | K2O |
| 206 | Potassium iodate formula | KIO3 |
| 207 | Potassium phosphate formula | KH2PO4 |
| 208 | Potassium sulfite formula | K2SO3 |
| 209 | Salicylic acid formula | C7H6O3 |
| 210 | Silicon dioxide formula | SiO2 |
| 211 | Silver acetate formula | AgC2H3O2 |
| 212 | Silver carbonate formula | Ag2CO3 |
| 213 | Silver chloride formula | AgCl |
| 214 | Silver nitrate formula | AgNO3 |
| 215 | Silver oxide formula | Ag2O |
| 216 | Silver phosphate formula | Ag3PO4 |
| 217 | Sodium acetate formula | C2H3NaO2 |
| 218 | Sodium bicarbonate formula | NaHCO3 |
| 219 | Sodium bromide formula | NaBr |
| 220 | Sodium thiosulfate formula | Na2S2O3 |
| 221 | Sodium carbonate formula | Na2CO3 |
| 222 | Sodium chloride formula | NaCl |
| 223 | Sodium chromate formula | Na2CrO4 |
| 224 | Sodium citrate formula | Na3C6H5O7 |
| 225 | Sodium cyanide formula | NaCN |
| 226 | Sodium dichromate formula | Na2Cr2O7 |
| 227 | Sodium fluoride formula | NaF |
| 228 | Sodium hydroxide formula | NaOH |
| 229 | Sodium hypochlorite formula | NaClO |
| 230 | Sodium iodide formula | NaI |
| 231 | Uric acid formula | C5H4N4O3 |
| 232 | Sodium nitrate formula | NaNO3 |
| 233 | Sodium nitride formula | Na3N |
| 234 | Sodium nitrite formula | NaNO2 |
| 235 | Sodium oxide formula | Na2O |
| 236 | Sodium peroxide formula | Na2O2 |
| 237 | Sodium phosphate formula | Na3PO4 |
| 238 | Sodium sulfate formula | Na2SO4 |
| 239 | Sodium sulfide formula | Na2S |
| 240 | Sodium sulfite formula | Na2SO3 |
| 241 | Strontium chloride formula | SrCl2 |
| 242 | Strontium nitrate formula | Sr(NO3)2 |
| 243 | Sucrose formula | C12H22O11 |
| 244 | Sugar formula | C12H22O11 |
| 245 | Sulfate ion formula | SO42− |
| 246 | Sulfur dioxide formula | SO2 |
| 247 | Sulfur trioxide formula | SO3 |
| 248 | Sulfuric acid formula | H2SO4 |
| 249 | Sulfurous acid formula | H2SO3 |
| 250 | Tartaric acid formula | C4H6O6 |
| 251 | Toluene formula | C7H8 |
| 252 | Urea formula | CH4N2O |
| 253 | Vinegar formula | C2H4O2 |
| 254 | Zinc acetate formula |
Zn(O2CCH3)2
|
| 255 | Zinc carbonate formula | ZnCO3 |
| 256 | Zinc chloride formula | ZnCl2 |
| 257 | Zinc hydroxide formula | Zn(OH)2 |
| 258 | Zinc iodide formula | ZnI2 |
| 259 | Zinc nitrate formula | Zn(NO3)2 |
| 260 | Zinc phosphate formula | Zn3(PO4)2 |
| 261 | Zinc sulfate formula | ZnSO4 |
| 262 | Zinc sulfide formula | ZnS |
Chemical Formula with Name
List of the first 108 chemical formulas for various compounds:
- H2O – Water
- NH3 – Ammonia
- CH4 – Methane
- CO2 – Carbon Dioxide
- O2 – Oxygen Gas
- H2 – Hydrogen Gas
- N2 – Nitrogen Gas
- NaCl – Sodium Chloride (Salt)
- HCl – Hydrochloric Acid
- H2SO4 – Sulfuric Acid
- HNO3 – Nitric Acid
- C6H12O6 – Glucose
- C12H22O11 – Sucrose (Table Sugar)
- CaCO3 – Calcium Carbonate
- Fe2O3 – Iron(III) Oxide (Rust)
- CO – Carbon Monoxide
- Cl2 – Chlorine Gas
- SO2 – Sulfur Dioxide
- H2O2 – Hydrogen Peroxide
- H2S – Hydrogen Sulfide
- NaOH – Sodium Hydroxide (Lye)
- C2H5OH – Ethanol (Alcohol)
- C6H5OH – Phenol
- C6H6 – Benzene
- C2H4 – Ethene
- C2H6 – Ethane
- CH3COOH – Acetic Acid
- HC2H3O2 – Acetate Ion
- NO2 – Nitrogen Dioxide
- SO4^2- – Sulfate Ion
- PO4^3- – Phosphate Ion
- O3 – Ozone
- H2CO3 – Carbonic Acid
- Na2CO3 – Sodium Carbonate
- MgSO4 – Magnesium Sulfate
- KNO3 – Potassium Nitrate
- Ca(OH)2 – Calcium Hydroxide
- FeSO4 – Iron(II) Sulfate
- Al2O3 – Aluminum Oxide
- NaHCO3 – Sodium Bicarbonate (Baking Soda)
- C6H5COOH – Benzoic Acid
- C4H10 – Butane
- C3H8 – Propane
- C2H2 – Ethyne (Acetylene)
- H2SiO3 – Silicic Acid
- H2PO4^- – Dihydrogen Phosphate Ion
- C2H3Cl – Vinyl Chloride
- C3H6O – Acetone
- Na2SO4 – Sodium Sulfate
- KMnO4 – Potassium Permanganate
- NaNO3 – Sodium Nitrate
- FeCl3 – Iron(III) Chloride
- NH4OH – Ammonium Hydroxide
- C6H4OH – Hydroquinone
- H2C2O4 – Oxalic Acid
- C6H5CH3 – Toluene
- C7H6O2 – Salicylic Acid
- C7H6O3 – Aspirin
- C8H10 – Xylene
- C6H5NH2 – Aniline
- C5H5N – Pyridine
- C6H5NO2 – Nitrobenzene
- H3PO4 – Phosphoric Acid
- Ca3(PO4)2 – Calcium Phosphate
- FeS – Iron(II) Sulfide
- NaClO – Sodium Hypochlorite
- CH3COCH3 – Acetophenone
- NaCN – Sodium Cyanide
- C6H5CHO – Benzaldehyde
- C6H5COCH3 – Acetophenone
- C2H4O2 – Acetic Anhydride
- CH3CH2COCH3 – Butanone (Methyl Ethyl Ketone)
- C6H5COCH2C6H5 – Acetylbenzene (Acetophenone)
- C6H5COOH – Benzoic Acid
- C6H5COC6H5 – Benzophenone
- C10H14N2 – Nicotine
- C6H5COC6H5 – Benzil
- C10H12O2 – Vanillin
- C10H14N2O – Caffeine
- C9H8O4 – Aspirin
- C12H10O4 – Terephthalic Acid
- C14H8O4 – Anthraquinone
- C12H8O4 – Resorcinol
- C15H10O4 – Hydroquinone
- C12H9N – Quinoline
- C10H7N – Indole
- C9H6N2O2 – Barbituric Acid
- C7H5N3O6 – TNT (Trinitrotoluene)
- C6H2(NO2)3CH3 – Tetryl
- C7H5N5 – Caffeine
- C6H5N3O9 – Nitroglycerin
- C6H12N2O4S2 – Thiamine (Vitamin B1)
- C6H8O6 – Ascorbic Acid (Vitamin C)
- C12H17N4O4S – Coenzyme A
- C10H16N2O3S – Acetyl-CoA
- C21H28O2 – Progesterone
- C21H30O5 – Cortisol
- C21H20O6 – Estrone
- C22H32O2 – Testosterone
- C19H28O2 – Prostaglandin E2
- C24H30O4 – Cholesterol
- C5H11NO2S – Cysteine
- C6H13NO2 – Arginine
- C5H9NO4 – Glutamine
- C6H13NO5 – Asparagine
- C9H11NO3 – Tyrosine
- C5H9NO4 – Aspartic Acid
- C3H7NO2 – Serine
Chemical Formula Importance for Class 11, 12 Students
Chemical formulas are important for a variety of reasons, including:
- Identifying chemicals: compounds can be recognized and set apart from other compounds using their chemical formulae. For instance, water has the chemical formula H2O, indicating that it is a mixture of two hydrogen atoms and one oxygen atom. This equation can be used to discriminate between water and other substances like sulfuric acid (H2SO4) or carbon dioxide (CO2).
- Calculating the mass of a compound: A compound’s chemical formula can be used to determine its mass. This is the case because the mass of a compound is equal to the sum of the masses of its atoms. One molecule of water, for instance, has an atomic mass of 18 amu, which is the result of adding the masses of two hydrogen atoms (1 amu each) and one oxygen atom (16 amu).
- Understanding the properties of a compound: A compound’s qualities can be understood by thinking about its chemical formula. For instance, we know that water is a polar molecule because its chemical formula (H2O) indicates that it has both a positive and a negative end. Many of water’s characteristics, including its high boiling point and propensity to dissolve ionic substances, are due to this characteristic.
- Designing new compounds: New compounds with desired qualities can be created using chemical formulae. For instance, a scientist can search for compounds with chemical formulas comparable to water if they wish to create a novel compound that is soluble in water.
Chemical All Formula Steps to Write?
Here are the steps on how to write a chemical formula:
- Identify the elements present in the compound. The chemical symbol for each element is used to represent it in a chemical formula. The chemical symbols are based on the Latin names of the elements.
- Determine the number of atoms of each element present in the compound. The number of atoms of each element is indicated by a subscript following the chemical symbol. If there is only one atom of an element, the subscript is not written.
- Write the chemical formula in the following order:
- Metals: Write the symbol for the metal first, followed by the subscript for the number of atoms of the metal.
- Nonmetals: Write the symbols for the nonmetals in alphabetical order, followed by the subscripts for the number of atoms of each nonmetal.
- Polyatomic ions: Write the symbol for the polyatomic ion, followed by the subscript for the number of ions.
- Check the formula to make sure that the charges are balanced. If the compound is ionic, the positive and negative charges must be balanced. This means that the sum of the positive charges must equal the sum of the negative charges.
रासायनिक सूत्र कक्षा 12- Chemical Formula with Name in Hindi
यौगिकों के लिए पहले 108 रासायनिक सूत्रों की सूची:
H2O – पानी
NH3 – अमोनिया
CH4 – मीथेन
CO2 – कार्बन डाइऑक्साइड
O2 – ऑक्सीजन गैस
H2 – हाइड्रोजन गैस
N2 – नाइट्रोजन गैस
NaCl – सोडियम क्लोराइड (नमक)
एचसीएल – हाइड्रोक्लोरिक एसिड
H2SO4 – सल्फ्यूरिक एसिड
HNO3 – नाइट्रिक एसिड
C6H12O6 – ग्लूकोज
C12H22O11 – सुक्रोज (टेबल शुगर)
CaCO3 – कैल्शियम कार्बोनेट
Fe2O3 – आयरन(III) ऑक्साइड (जंग)
सीओ – कार्बन मोनोऑक्साइड
सीएल2 – क्लोरीन गैस
SO2-सल्फर डाइऑक्साइड
H2O2 – हाइड्रोजन पेरोक्साइड
H2S – हाइड्रोजन सल्फाइड
NaOH – सोडियम हाइड्रॉक्साइड (लाइ)
C2H5OH – इथेनॉल (अल्कोहल)
C6H5OH – फिनोल
C6H6 – बेंजीन
C2H4 – एथीन
C2H6 – इथेन
CH3COOH – एसिटिक अम्ल
HC2H3O2 – एसीटेट आयन
NO2 – नाइट्रोजन डाइऑक्साइड
SO4^2–सल्फेट आयन
PO4^3- – फॉस्फेट आयन
O3 – ओजोन
H2CO3 – कार्बोनिक एसिड
Na2CO3 – सोडियम कार्बोनेट
MgSO4 – मैग्नीशियम सल्फेट
KNO3 – पोटेशियम नाइट्रेट
Ca(OH)2 – कैल्शियम हाइड्रॉक्साइड
FeSO4 – आयरन(II) सल्फेट
Al2O3 – एल्यूमिनियम ऑक्साइड
NaHCO3 – सोडियम बाइकार्बोनेट (बेकिंग सोडा)
C6H5COOH – बेंजोइक एसिड
C4H10 – ब्यूटेन
C3H8 – प्रोपेन
C2H2 – एथाइन (एसिटिलीन)
H2SiO3 – सिलिकिक एसिड
H2PO4^- – डाइहाइड्रोजन फॉस्फेट आयन
C2H3Cl – विनाइल क्लोराइड
C3H6O – एसीटोन
Na2SO4 – सोडियम सल्फेट
KMnO4 – पोटेशियम परमैंगनेट
NaNO3 – सोडियम नाइट्रेट
FeCl3 – आयरन(III) क्लोराइड
NH4OH – अमोनियम हाइड्रॉक्साइड
C6H4OH – हाइड्रोक्विनोन
H2C2O4 – ऑक्सालिक एसिड
C6H5CH3 – टोल्यूनि
C7H6O2 – सैलिसिलिक एसिड
C7H6O3 – एस्पिरिन
C8H10 – ज़ाइलीन
C6H5NH2 – एनिलीन
C5H5N – पाइरीडीन
C6H5NO2 – नाइट्रोबेंजीन
H3PO4 – फॉस्फोरिक एसिड
Ca3(PO4)2 – कैल्शियम फॉस्फेट
FeS – आयरन (II) सल्फाइड
NaClO – सोडियम हाइपोक्लोराइट
CH3COCH3 – एसिटोफेनोन
NaCN – सोडियम सायनाइड
C6H5CHO – बेंजाल्डिहाइड
C6H5COCH3 – एसिटोफेनोन
C2H4O2 – एसिटिक एनहाइड्राइड
CH3CH2COCH3 – ब्यूटेनोन (मिथाइल एथिल कीटोन)
C6H5COCH2C6H5 – एसिटाइलबेन्जीन (एसिटोफेनोन)
C6H5COOH – बेंजोइक एसिड
C6H5COC6H5 – बेंज़ोफेनोन
C10H14N2 – निकोटीन
C6H5COC6H5 – बेंज़िल
C10H12O2 – वैनिलिन
C10H14N2O – कैफीन
C9H8O4 – एस्पिरिन
C12H10O4 – टेरेफ्थेलिक एसिड
C14H8O4 – एन्थ्राक्विनोन
C12H8O4 – रेसोरिसिनोल
C15H10O4 – हाइड्रोक्विनोन
C12H9N – क्विनोलिन
C10H7N – इंडोल
C9H6N2O2 – बार्बिट्यूरिक एसिड
C7H5N3O6 – टीएनटी (ट्रिनिट्रोटोलुइन)
C6H2(NO2)3CH3 – टेट्रिल
C7H5N5 – कैफीन
C6H5N3O9 – नाइट्रोग्लिसरीन
C6H12N2O4S2 – थायमिन (विटामिन B1)
C6H8O6 – एस्कॉर्बिक एसिड (विटामिन सी)
C12H17N4O4S – कोएंजाइम ए
C10H16N2O3S – एसिटाइल-सीओए
C21H28O2 – प्रोजेस्टेरोन
C21H30O5 – कोर्टिसोल
C21H20O6 – एस्ट्रोन
C22H32O2 – टेस्टोस्टेरोन
C19H28O2 – प्रोस्टाग्लैंडीन E2
C24H30O4 – कोलेस्ट्रॉल
C5H11NO2S – सिस्टीन
C6H13NO2 – आर्जिनिन
C5H9NO4 – ग्लूटामाइन
C6H13NO5 – शतावरी
C9H11NO3 – टायरोसिन
C5H9NO4 – एस्पार्टिक एसिड
C3H7NO2 – सेरीन



 JEE Mains Result 2025 Session 2 Live: Sc...
JEE Mains Result 2025 Session 2 Live: Sc...
 UP, MP, CBSE Board Result 2025 Live Upda...
UP, MP, CBSE Board Result 2025 Live Upda...
 [Live] CUET UG Date Sheet 2025 @cuet.nta...
[Live] CUET UG Date Sheet 2025 @cuet.nta...










