Table of Contents
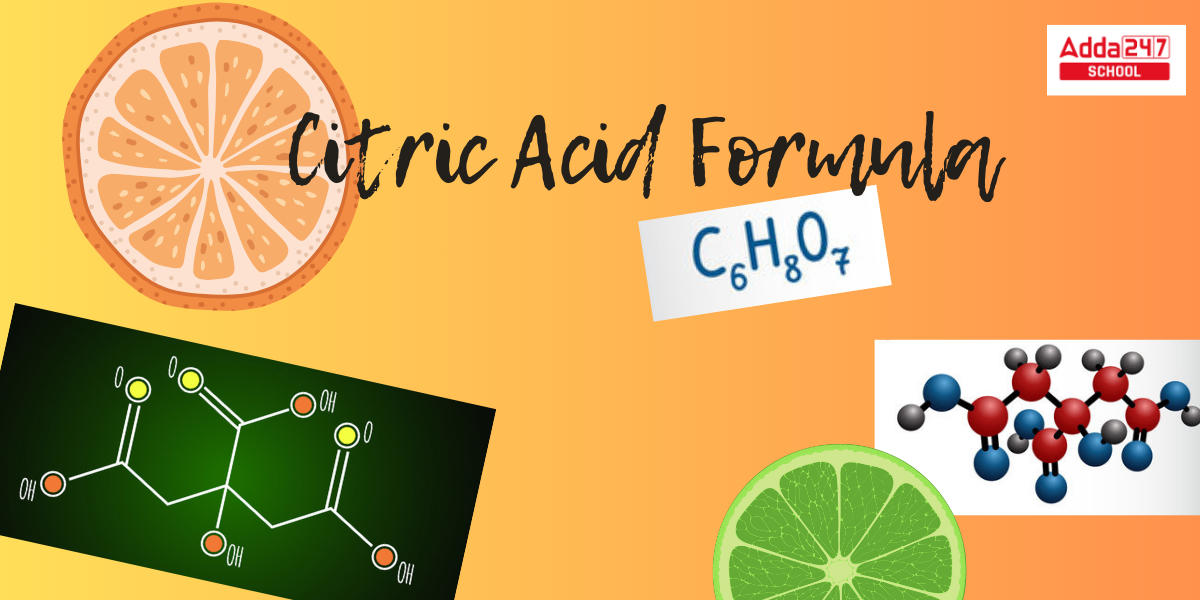
Citric Acid Formula: Citric acid is found in citrus fruits and plants all around us. Even animals have been observed with traces of it in their bodies. C6H8O7 is the Citric Acid formula. Citrus fruits, such as oranges and lemons, have a sour flavor due to the presence of citric acid, which accounts for 8% of their total dry weight. Citric acid is a weak organic acid with a powerful organic compound. To preserve processed foods, manufacturers add artificial citric acid. This article will go through the Citric Acid formula, structure, characteristics, IUPAC name, preparation, uses, and more.
Citric Acid Formula
Carl Wilhelm Scheele, a scientist, discovered this organic acid for the first time in 1784. It has no scent and a sour taste, and it seems like a white crystalline solid. Its crystal structure is monoclinic. It is similar to table salt from the start and is offered in the market as sour salt. Citric acid is utilized in various medicines, drinks, disinfectants, and cosmetic products due to its numerous benefits. Citric acid is an important molecule in biochemistry because it serves as an intermediary in the citric acid cycle. As a result, it happens in practically all living things’ digestion. It also functions as a natural antioxidant and cleaning agent.
What is the Citric Acid Formula?
As we mentioned earlier, the chemical citric acid formula is C6H8O7. Citric acid has 6 carbon atoms and 8 hydrogen atoms. At the same time, it contains seven oxygen atoms. If you wish to figure out the extended formula of Citric acid that is – CH2 COOH – COHCOOH-CH2COOH, use the above ratios. Citric acid is vital in biochemistry as a step in the citric acid cycle and hence occurs in practically every living organism’s metabolism.
Citric Acid Formula IUPAC Name
Citric acid belongs to the carboxylic acid family. The citric acid IUPAC name is 2-hydroxypropane-1,2,3-tricarboxylic acid. Citric acid is formed by condensing acetyl CoA and oxaloacetic acid. Furthermore, the chemical reaction at the Ketone carbon transfers the acetyl group CH3COO from CoA to oxaloacetic acid. This reaction tries to join two carbon atoms with four carbon atoms, resulting in the formation of an acid with six carbon atoms. This is referred to as acid synthesis.
Citric Acid Properties
Citric acid is essential for creating energy in the human body and keeping the biosynthetic reaction going. Citric acid also acts as a sugar breaker in glycolysis, providing energy for ATP generation in the process. Citric acid has the following important properties.
- It is a crystalline substance that is colorless, odorless, and tasteless.
- Citric Acid Formula – C6H8O7.
- Citric acid crystals have a monoclinic form.
- Citric acid has a molecular weight/molar mass of 192.124 g/mol.
- The density of Citric Acid -1.66 g/cm3
- Citric Acid is soluble in ether, alcohol, and acetone in addition to water. Citric acid has a boiling point of 310 °C.
- Citric acid has a melting point of 153 degrees Celsius.
- It exists as a white crystalline powder at ambient temperature.
Citric Acid Formula Stucture
Citric acid crystals are white in their natural state, as seen on the tops of sour candies. To begin, the chemical formula for citric acid is C6H8O7, where (C) represents carbon, (H) represents hydrogen, and (O) represents oxygen. At the same time, the molecular formula for citric acid is CH2COOH-C(OH)COOH-CH2COOH. Citric acid has various aliases, including 2-hydroxypropane-1,2,3-tricarboxylic acid, Anhydrous, and Anhydrous citric acid.Let’s learn about the Citric Acid Stucture using the representation below.
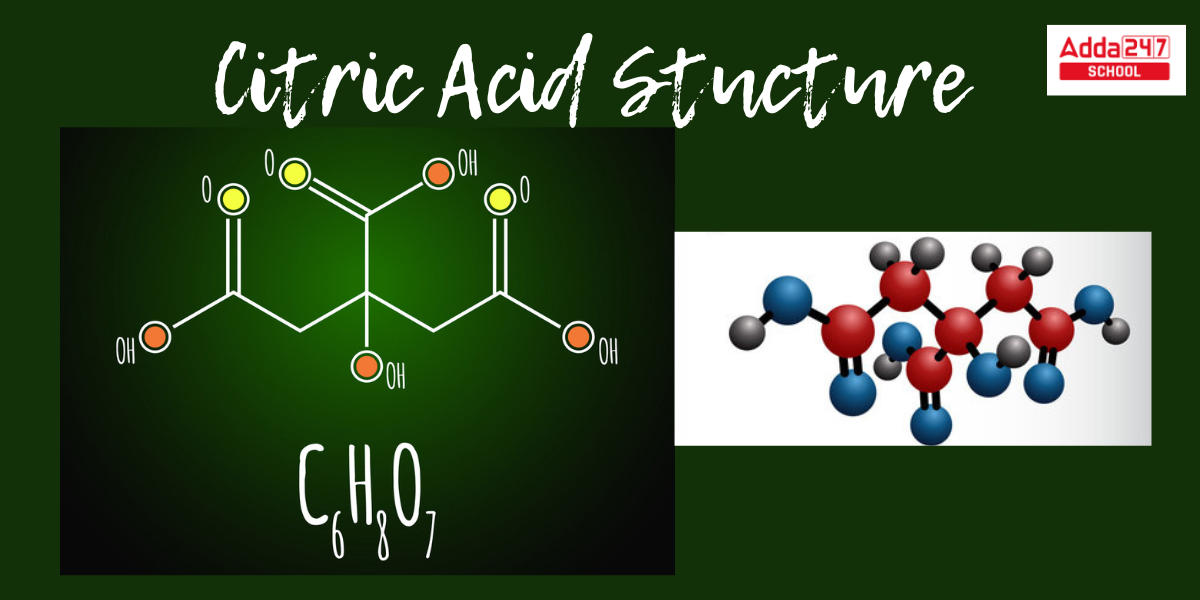
Citric Acid Preparation
The Citric Acid Preparation through biological and laboratory techniques are discussed below.
- Citric acid is created solely through fermentation with the filamentous fungus Aspergillus niger.
- Carbohydrates or agro-industrial wastes are used as substrates in industrial production via three processes: submerged, surface, and solid fermentations.
- Sucrose is utilized in the production of citric acid. When the resultant solution is filtered out of the mold, citric acid is separated by reacting it with calcium hydroxide (lime) to produce a salt of calcium citrate salt.
- Treatment with sulfuric acid restores citric acid from the previously generated calcium citrate salt.
- Citric acid is sometimes extracted from the fermentation broth. An extraction procedure is used to separate it.
- Citric acid is isolated from the organic base triarylamine using a hydrocarbon-based solution.
- Following that, water is used to re-extract the organic solution.
Citric Acid (C6H8O7) Uses
Citric acid is naturally present in many vegetables and fruits. It has numerous applications due to its characteristics. Some of them are outlined below:
- Citric acid is widely used in food and beverages. It imparts a citrus essence to the cuisine and enhances its flavor.
- Citric acid has long been used to preserve food, and many canned food products include a white layer of Citric acid in order to keep them fresh in a can for longer.
- It lowers the pH of frozen meals and makes the oxidative enzymes sluggish. As a result, citric acid in food inhibits bacterial development and eventually degrades it. Additionally, some ice cream producers favour employing citric acid as an emulsifier to prevent the formation of fat globules.
- In caramel, citric acid is used to crystallize sugar.
- It is used to make detergents and soaps, as well as to soften acidic water. Citric acid is used in leather tanning and electroplating.
- Citric acid is used in numerous cosmetic products due to its pH-balancing properties.
- This acid is also utilized in the storage of blood in hospitals. Because of its anticoagulant properties, it aids in calcium chelation in the blood.
- It aids in the flow of calcium and vitamin C to the body, hence aiding in bone preservation.
- Increasing antioxidant qualities in Citric acid aid in the prevention of diseases such as osteoporosis and bone health.


 TS Inter Results 2025 Date for TSBIE 1st...
TS Inter Results 2025 Date for TSBIE 1st...
 CUET Accountancy Chapter Wise Weightage ...
CUET Accountancy Chapter Wise Weightage ...
 CUET UG Exam Date Sheet 2025 @cuet.nta.n...
CUET UG Exam Date Sheet 2025 @cuet.nta.n...










