The Class 10 Science Expected Board Exam Paper 2024 will help students score more marks in the actual board exam 2024. Science is an application-oriented subject that requires comprehensive practice to understand it. For this purpose, the expected board paper 2024 for the Class 10 science subject is even more important to practice.
The expected board paper has been designed based on the latest exam pattern. Along with this, the answers to the questions provided in the expected board exam paper have also been provided below. It will help students learn the answer writing technique as per the current marking scheme for class 10 science.
Class 10 Science Board Exam Paper 2024
Expected Class 10 Science Board Question Paper 2024: The CBSE Class 10 Science Examination will be held on March 2, 2024. With just a few months left for the exam, it is imperative to put all the efforts. Therefore now is a key moment to enhance our Class 10 Science exam result. Students should practice the Sample question papers for Science as often as possible; this will help them face the questions in the examination hall.Our Adda247 Expert faculty prepares the Expected expected Class 10 Board Science Exam Paper 2024 for the benefit of class 10 candidates, which is based on the genuine CBSE Class 10 Exam pattern. Students who are going to take the class 10 board exam in 2024, must solve the Expected Class 10 Board Science Exam Paper 2024. Students should carefully go through the solutions of the expected board paper too, in order to understand the answer writing techniques.
Science Class 10 Sample Paper 2024
Apart from the expected board paper 2024, students must also solve the official sample paper 2024 provided by the CBSE for the class 10 science subject. It will help students cover a wide range of questions that are important for the board exam 2024. Our expert has also provided the solutions of the sample paper 2024 so that students can know the perfect answers to the provided questions. The CBSE Class 10 Science Sample Paper 2024 with solutions is given below.
Science Class 10 Board Exam Paper 2024- Expected
Adda247’s Class 10 Board Science Exam Paper 2024, like the official CBSE Class 10 Science sample paper, has 39 questions in 5 sections for an overall score of 80. Check the mark distribution for the Class 10 Board Science Exam Paper 2024 below.
- Section A consists of 20 objective-type questions carrying 1 mark each.
- Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these
questions should in the range of 30 to 50 words. - Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should in the range of 50 to 80 words
- Section D consists of 3 Long Answer type questions carrying 05 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.
Expected Class 10 Board Science Exam Paper 2024 Section A
SECTION – A
Select and write one most appropriate option out of the four options given for each of the questions 1 – 20.Each Questions carries 1 Mark.
Q1 A complete circuit is left on for several minutes, causing the connecting copper wire to become hot. As the temperature of the wire increases, the electrical resistance of the wire
(a) Increases
(b) Decreases
(c) Remains constant
(d) None of the above
Q2 Carbon atom attains its stability by sharing its valance electron in covalent bond sharing. The number of electrons that the carbon atom shares is/are
(a) 1
(b) 2
(c) 3
(d) 4
Q3 Diksha was playing with her friend Pragati with some wires, batteries, and a magnetic compass. Pragati was trying to find the direction of the magnetic north of the Earth so she put the compass on the ground steadily but Diksha connects a battery to the wire and put the wire over the compass. The compass deflects from the magnetic north of the Earth. The reason was
(a) Magnetic effect due to current carrying wire
(b) Gravitational effect due to current carrying wire
(c) Electric effect due to current carrying wire
(d) Mechanical effect due to current carrying wire
Q4 Name a device that helps to maintain a potential difference across a conductor.
(a) Ammeter
(b) Voltmeter
(c) Cell
(d) Resistor
Q5 Fe2O3 + 2Al —-→ Al2O3 + 2Fe
The above reaction is an example of an
(a) combination reaction.
(b) double displacement reaction.
(c) decomposition reaction.
(d) displacement reaction.
Q6 Food is synthesized from simple inorganic raw materials such as CO2 and water.
(a) Heterotrophic Nutrition
(b) Autotrophic Nutrition
(c) Both (a) and (b)
(d) neither (a) nor (b)
Q7
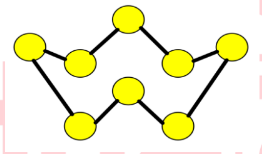
This is a structure of
(a) Sulphur Molecule
(b) Carbon Molecule
(c) Iron molecule
(d) Helium molecule
Q8 The number of isomeric structures are possible for Bromopentane
(a) 1
(b) 3
(c) 8
(d) 4
Q9 The sex chromosomes present in a female gamete is
(a) XX
(b) XY
(c) YY
(d) YZ
Q10 Sunita was trying to measure the resistance of a wire using a very sensitive reading device. She measures it and finds that the resistance of the wire is 1Ω. Her friend took that wire and stretched it so its length increases as well as its diameter decreases. The resistance of the wire has become
(a) More than 1Ω
(b) Less than 1Ω
(c) Equal to 1Ω
(d) 100 Ω
Q11 Mirrors used by dentists and for makeup are
(a) Concave, Convex
(b) Convex, Concave
(c) Concave, Concave
(d) Convex, Convex
Q12 A ray of light is passing through the centre of curvature gets reflected along the same path. The mirror is used in this case is
(a) Convex
(b) Concave
(c) Plane
(d) It’s a lens
Q13 When resistors of same value are joined in parallel combination, the total resistance value of the construction will
(a) Increase
(b) Remains constant
(c) Decrease
(d) Increase then decrease
Q14 The resistance of an ideal Ammeter and a Voltmeter is
(a) 0Ω ,∞Ω
(b) ∞Ω, 0Ω
(c) 0Ω ,0Ω
(d) ∞Ω,∞Ω
Q15 The formation of sperms in testes and ova is called
(a) Ovulation
(b) Gametogenesis
(c) Fertilization
(d) None of the above
Q16 Involuntary actions controlled by the hindbrain are
(a) Vomiting
(b) Salvation
(c) Heart beat
(d) All of the above
Q17 The secretion of which hormone is responsible for the dramitic changes in appearance in girls when they hit puberty
(a) Oestrogen
(b) Testosterone
(c) Thyroid
(d) Spinal cord
Q18 When the leaf of a touch me not plant (Mimosa pudica) is touched, the electrical signal send signals from leaflets to pulvinars (petiole) of leaf. The movement due to the difference of turgidity of the cells is known as
(a) Relaxed movement
(b) Turgor Movement
(c) Stiff movement
(d) None of the above
Q19 When a white beam of light passed through a glass prism, it disperses into its constitute colours. The least and the most deviated lights are
(a) Red, Violet
(b) Violet, Red
(c) Red, Yellow
(d) Yellow, Green
Q20 Consider the following reaction Which of these two reactions will take place?

(a) Only (i) —
(b) Only (ii)
(c) Both (i) and (ii)
(d) Neither (I) nor (ii)
Class 10 Board Science Exam Paper 2024 -Section B
SECTION – B
Q. no. 21 to 26 are very short answer questions and Each Question carries 2 Marks
Q21 List three factors on which the resistance of a conductor depends.
Q22 Covalent compounds have low melting and boiling point. Why?
Q23 Draw ray diagrams for the following cases when a ray of light passing through center of curvature of a concave mirror is incident on it.
Q24 List any two steps involved in sexual reproduction and write its two advantage.
Q25 State the reason why carbon can neither form C4+ cations nor C4- anions, but forms covalent compounds.
Q26 Write one point of difference between a hydrated salt and an anhydrous salt.
Class 10 Science Board Expected Test Paper 2024 Section C
SECTION – C
Q. no. 27 to 33 are short answer questions. Each Question carries 3 marks
Q27 State the changes that take place in the uterus when:
(a) Implantation of embryo has occurred.
(b) Female gamete/egg is not fertilised.
Q28 State three factors on which the strength of magnetic field produced by a current carrying solenoid depends.
Q29 What is a gene? Explain heredity. All the variations in a species do not have equal chances of survival. Why?
Q30 Write the electronic configuration of two elements X and Y whose atomic numbers are 20 and 17 respectively. Write the molecular formula of the compound formed when element X reacts with element Y. Draw electron-dot of structure of the product and also state the nature of the bond formed between both the elements.
Q31 Write the function of each of the following parts of human eye:
(i) Cornea
(ii) Iris
(iii) Crystalline lens
Q32 What is water of crystallization? Write the common name and chemical formula of a commercially important compound which has ten water molecules as water of crystallization.
Q33 Design an activity to demonstrate that a bar magnet has a magnetic field around it.
Class 10 Board Science Exam Paper 2024-Section D
SECTION – D
Q. no. 34 to 36 are long answer questions. Each Question carries 4 marks
Q34 In the following food chain, plants provide 500 J of energy to rats. How much energy will be available to hawks from snakes? List two biotic components of a biosphere.
Q35 Soaps and detergents are both types of salts. State the difference between the two. Write the mechanism of the cleansing action of soaps. Why do soaps not form lather (foam) with hard water? Mention any two problems that arise due to the use of detergents instead of soaps.
Q36 List three techniques that have been developed to prevent pregnancy. Which one of these techniques is not meant for males? How does the use of these techniques have a direct impact on the health and prosperity of a family?
Science Class 10 Question Paper 2024-Section E
SECTION – E
Q. no. 37 to 39 are case–based/data-based questions with 2 to 3 short sub-
parts. Internal choice is provided in one of these sub-parts. Each Question carries 5 marks
Q37 Read the following and answer any four questions from ( i ) to (v)
In an experiment, a scientist removed some cells from the growing point of a plant and placed it a suitable medium containing nutrients and plant hormones leading to the formation of shapeless lump or mass called X. X is then transferred to another medium which lead to development of roots. X with developed roots is then transferred into another medium that induced the development of shoots. X in this way differentiated into tiny plantlets which were transplanted into pots where they grew into mature plants.
(i) What is X in the given paragraph?
(a) Plantlet
(b) Callus
(c) Embryoid
(d) Tissue
(ii) Which technique has the scientist used for propagation of plant ?
(a) Layering
(b) Grafting
(c) Tissue culture
(d) Cutting
(iii) What is the advantage of the technique mentioned in the paragraph ?
(a) It helps in production of disease free plant
(b) It Is very fast technique as thousands of plantlets can be produced in a few weeks of time.
(c) It is also known as micropropagation due to extremely small amount of plant material used for propagation.
(d) All of these
(iv) Select the incorrect statement regarding the propagation technique mentioned in the paragraph
(a) It is used in the production of ornamental plants like orchids, Carnation etc.
(b) It is a modern method of artificial propagation of plant
(c) Plants produced by this method are genetically different from the parent
plant
(d) Very little space is needed for developing new plants by this technique.
Q38 The heart is a muscular organ which is as big as our fist. Because both oxygen and carbon dioxide have to be transported by the blood, the heart has different chambers to prevent the oxygen-rich blood from mixing with the blood containing carbon dioxide. The carbon dioxide rich blood has to reach the lungs for the carbon dioxide to be removed, and the oxygenated blood from the lungs has to be brought back to the heart. This oxygen-rich blood is then
pumped to the rest of the body.
i) How many chambers are present in the heart of mammals and reptiles?
ii) Who carry deoxygenated blood from body to heart?
iii) What do you meant by the term double circulation?
iv) What is hypertension?
V) Which device measured blood pressure?
Q39 The electrical energy consumed by an electrical appliance is given by the product of its power rating and the time for which it is used. The SI unit of electrical energy is Joule. Actually, Joule represents a very small quantity of energy and therefore it is inconvenient to use where a large quantity of energy is involved. So for commercial purposes we use a bigger unit of electrical energy which is called kilowatt hour. 1 kilowatt-hour is equal to 3.6 x 106 joules of electrical energy.
(i) The energy dissipated by the heater is E. When the time of operating the heater is doubled, the energy dissipated is
(a) doubled
(b) half
(c) remains same
(d) four times
(ii) The power of a lamp is 60 W The energy consumed in 1 minute is
(a) 360J
(b) 36J
(c) 3600J
(d) 3.6 J
(iii) The electrical refrigerator rated 400 W operates 8 hours a day. The cost of electrical energy is ₹5 per kWh. Find the cost of running the refrigerator for one day?
(a) ₹32
(b) ₹16
(c) ₹8
(d) ₹4
(iv) Calculate the energy transformed by a 5 A current flowing through a resistor of 2Ω for 30 minutes?
(a) 90 kJ
(b) 80 kJ
(c) 60 kJ
(d) 40 kJ
Science Class 10 Board Question Paper 2024- With Solutions
We have also provided you with detailed solutions for Science Class 10 Board Question paper 2024. So, after solving the Expected Class 10 Board Science Exam Paper 2024, students can analyze their scores. It will help students perform better in the actual board exam paper 2024 of the science subject.
| Class 10 Expected Science Exam Paper 2024 With Solutions Download |
Most Important Questions for Class 10 Science Board Exam 2024
Here we discussed all-important question of class 10 is given below mark wise.
Most important questions for class 10 science board exam 2024 for 2 Marks
Chapter – 4 Carbon and its Compounds
- The element carbon forms a very large number of compounds. Give reason for this fact.
- Covalent compounds generally don’t conduct electricity.Why?
- State two characteristic features of carbon which when put together give rise to large number of carbon compounds.
- What is catenation?
- What do you mean by tetravalency of carbon?
- Define structural isomerism. Draw the structures of two isomers of butane.
Chapter – 5 Periodic Classification of Elements
- How does the tendency to lose electrons change in a group and why?
- Why He, Ne and Ar are called inert gases?
- Write two limitations of Mendeleev’s Periodic Table.
- Why is the position assigned to hydrogen in the periodic table considered anomalous?
- How does the metallic character of an element vary as we go down a group? Give reason for this variation.
- Why metallic oxides are basic in nature whereas non-metallic oxides are acidic in nature?
- How does the atomic size vary as we go down a group? Write the reason behind it.
- Four elements P, Q, R and S have atomic number 12, 13, 14 and 15 respectively. Answer the following:
(a) Classify these elements as metals and non-metals.
(b) Which of these elements will form the most basic oxide?
- (a) How do we calculate the valency of an element from its electronic configuration?
(b) How does the valency vary in a period?
- How many groups and periods are present in the Modern Periodic Table?
Chapter – 8 How Do Organisms Reproduce?
- Write two important functions of testosterone.
- What is placenta ? Also write its functions.
- Why do we see different types of organisms around us?
- What is the importance of variation?
- Why is vegetative propagation practiced for growing some types of plants?
- Write names of male and female sex hormones.
- Mention the parts of a flower.
- What is the effect of DNA copying which is not perfectly accurate on the reproduction process?
Chapter – 9 Heredity and Evolution
- Explain sex determination.
- What are genes? Where are they located?
- What is meant by dominant genes and recessive genes? Give one example of each.
- What are sex chromosomes?
- Distinguish between autosomes and sex chromosomes.
- Name the four blood groups in human.
- Why did Mendel choose pea plants for conducting his experiments on inheritance?
- What does law of segregation states?
- Differentiate between somatic variation and genetic variation?
- Write down the phenotypic ratio and genotypic ratio in monohybrid cross.
Chapter – 12 Electricity
- What is electrical resistivity of a material? Write its unit.
- What is the commercial unit of electric energy? Convert it into joules.
- State Ohm’s law. Write the necessary conditions for its validity.
- (a) Define electric power. Express it in terms of potential difference V and resistance R.
- An electrical fuse is rated at 2 A. What is meant by this statement?
- State difference between the wire used in the element of an electric heater and in a fuse wire.
Chapter – 13 Magnetic effects of current
- What is meant by solenoid?
- What is an electromagnet? What is the direction of magnetic field lines?
- What is the shape of magnetic field lines due to a straight current carrying conductor?
- Why do magnetic field lines not intersect each other?
- How is the strength of magnetic field near a straight current carrying conductor related to the strength of current in the conductor?
- Write one application of each of the following:
(a) Fleming’s left hand rule
(b) Fleming’s right hand rule
- List two properties of magnetic field lines.
- When is the force experienced by a current-carrying conductor placed in a magnetic field largest?
Chapter – 15 Our Environment
- Explain how does making of Kulhads affect our environment?
- State two differences between a consumer and producer.
- Draw the line diagram showing flow of energy in an ecosystem.
- Define a food web. State its significance for ecosystem.
- What are phytoplanktons.
- What will happen if all the phytoplanktons are eliminated from pond?
- What is an ecosystem? List its two main components.
- Name two natural ecosystems.
- We do not clean ponds or lakes, but an aquarium needs to be cleaned regularly. Explain.
- In the following food chain 20J of energy was available to the hawks. How much would have been present in the plants?
Plants → Rats → Snakes → hawks.
Most important questions for class 10 science board exam 2024 for 3 Marks
Chapter 4: Carbon and its Compounds
- What is a homologous series? List any of its two features.
- Give reasons for the following:
(a) Element carbon forms compounds mainly by covalent bonding.
(b) Diamond has a high melting point.
(c) Graphite is a good conductor of electricity.
- Explain why carbon atom is unable to form either cation or anion?
- Two carbon atoms cannot be linked to each other by more than three covalent bond. Why?
- Describe the two properties of carbon which lead to the formation of huge number of compounds.
Chapter 5: Periodic Classification of Elements
- Elements A,B,C,D and E having atomic number: 4, 9, 14, 19, 20.
(a) Name the elements having same valence electrons.
(b) Name the elements of same period. Give reason to your answer.
(c) Name the elements of same group. Give reason to your answer.
- (a) Newland, Mendeleev and Dobereiner gave their contribution in building Modern Periodic table. List one merit and one demerit of their contribution.
(b) Write the Modern Periodic law.
- In reference to Modern periodic table explain what is meant by periodic function of properties of elements? What is the effect on the power of accepting electrons on moving from left to right in Periodic table.
- X and Y are two elements having atomic number 20 and 17. Write the formula of compound formed by X and Y and also draw electron dot structure. Write the nature and bond in XY.
- Electronic configuration of an element is 2, 8, 47.
(a) Write the group number of this element in modern periodic table.
(b) Write its name and one physical property of this element.
- An element belongs to third period and group 13. Find its valence electrons and valency. Another element Y has 18 neutrons in its nucleus and Mass Number 35. Write the group and period number of element Y.
- Write the name, symbol and electronic configuration of an element having atomic number 11. Write the group and period number of this element.
Chapter 8: How Do Organisms Reproduce?
- What is placenta? Write its functions.
- What is the importance of variation?
- Why is vegetative propagation practiced for growing some types of plants?
- Differentiate between bisexual and unisexual flowers.
- What is tissue culture?
- Explain the process of fertilisation in flowering plants.
- Name the different constituents of semen.
- Draw a well-labelled diagram of male reproductive system.
- What is pre-natal sex determination? Why is it banned?
- Draw a labelled diagram of the longitudinal section of a flower.
Chapter 9: Heredity and Evolution
- Differentiate between homologous and analgous organs, with examples.
- Variation is beneficial to the species but not necessarily for the individual. Give three reasons to justify it.
- The human hand, cat paw and horse foot, when studied in detail show the same structure of bones and point towards a common origin.
(a) What do you conclude from this?
(b) What is the term given to such structure?
- What is genetic drift. Explain with examples.
- Write a short note on (a) gene flow (b) Natural selection
- Distinguish between autosomes and sex chromosomes.
- Distinguish between inherited traits and acquired traits giving one example of each. Give reason why the traits acquired by an individual during the life time are not inherited.
- A cross is carried between pure bred tall plant and pure bred dwarf pea plant.
(a) What is the phenotype of F1 progeny and why
(b) What is the phenotype of F2 progeny when F1 is selfed.
Chapter 12: Electricity
- Find a relationship between P, I and V.
- State Ohm’s law. Derive relation between I, V and R. Draw the graph between V and I.
- What is Joule’s heating effect of current P? Derive its expression.
- What would be new resistance if length of conductor is doubled and thickness is halved?
- Find the effective resistance between A and B.
- Which is the better way to connect lights and other appliances in domestic wiring and why ?
- Show how would you join three resistors, each of resistance 9 Ω so that the equivalent resistance of the combination is (i) 13.5 Ω, ii) 6 Ω?
- (a) Write Joule’s law of heating.
(b) Two lamps, one rated 100 W; 220 V, and the other 60 W; 220 V, are connected in parallel to electric mains supply. Find the current drawn by two bulbs from the line, if the supply voltage is 220 V.
- (a) List the factors on which the resistance of a conductor in the shape of a wire depends.
(b) Why are metals good conductors of electricity whereas glass is a bad conductor of electricity?
(c) Why are alloys commonly used in electrical heating devices?
Chapter 13: Magnetic Effects of Current
- A charged particle enters at right angle into a uniform magnetic field. What is the nature of charge particle if it experiences a force in a direction pointing vertically out of page.
- Write the three ways to produce magnetic field.
- What is solenoid ? Where the magnetic field is uniform in solenoid?
- Draw the pattern of magnetic field lines due to current carrying straight conductor.
- (a) Draw a diagram to show the pattern of magnetic field lines through and around a current carrying solenoid.
(b) List two factors on which the strength of the magnetic field produced by the solenoid depends.
(c) What is the effect of placing an iron core in a solenoid?
- A coil of insulated copper wire is connected to a galvanometer. What will happen if a bar magnet is
(a) pushed into the coil,
(b) withdrawn from inside the coil,
(c) held stationary inside the coil?
- (a) Mention the factors on which the direction of force experienced by a current-carrying conductor placed in a magnetic field depend.
(b) Under what condition is the force experienced by a current-carrying conductor placed in a magnetic field maximum?
(c) A proton beam is moving along the direction of a magnetic field. What force is acting on proton beam?
Chapter – 15 Our Environment
- Why only 10% energy is transferred to the next trophic level?
- Why is ozone layer important for the existence of life on earth?
- What is the role of decomposers in ecosystem?
- Draw an energy pyramid showing different trophic levels.
- How ozone molecule is formed in the atmosphere?
- Why natural ecosystem is more stable than artificial ecosystem?
- Why some materials are not decomposed by the action of micro-organisms? Give any two ways in which non-biodegradable wastes would affect the environment.
- What is a food web? Explain with example.
- How the components of an ecosystem are dependent on each other?
Most important questions for class 10 science board exam 2024 for 4 Marks
Chapter – 4 Carbon and its compounds
- Read the following carefully.
In covalent compounds atoms share valence electrons to satisfy the octet. Each atom shares one pair or two pairs or three pairs of electrons depending on their combining capacity. In electron dot structures only number of valence electrons are shown around the symbols of constituent atoms. Carbon using its valency of four can make either single, double or triple bonds with other carbon atoms or any other atoms. Carbons self-linking property is called catenation. In hydrocarbons carbon makes aliphatic or cyclic molecules they are either saturated or unsaturated. Based on these facts Read the following paragraph and answer the questions given below. An element X combines with Y to form a colourless odourless gas, Z which turns lime water milky is the major constituent of all organic molecules. Five X atoms combines with hydrogens to form a cyclic saturated hydrocarbon J and aliphatic unsaturated hydrocarbon Q.Q is used in gas welding.
(a) Identify compound Z and draw its electron dot structure.
(b) Write the chemical formula and IUPAC name of compound Q
(c) What is the common name of Q
(d) How many single covalent bonds are present in compound J?
Answer:
- a) Z is CO2 its electron dot structure is
- b) C2H2,ethyne
- c) Acetylene
- d) 15
Q. Read the following and answer questions from (i) to (iv)
The compounds which have the same molecular formula but differ from each other in physical or chemical properties are called isomers and the phenomenon is called isomerism. When the isomerism is due to difference in the arrangement of atoms within the molecule, without any reference to space, the phenomenon is called structural isomerism. In other words. Structural isomers are compounds that have the same molecular formula but different structural formulas, i.e., they are different in the order in which different atoms are linked. In these compounds, carbon atoms can be linked together in the form of straight chains,branched chains or even rings.
(i) Which of the following sets of compounds have same molecular formula?
(a) Butane and iso-butane
(b) Cyclohexane and hexene
(c) Propanal and propanone
(d) All of these
(ii) In order to form branching, an organic compound must have a minimum of
(a) four carbon atoms
(b) three carbon atoms
(c) five carbon atoms
(d) any number of carbon atoms.
(iii) Which of the following is an isomeric pair?
(a) Ethane and propane
(b) Ethane and ethene
(c) Propane and butane
(d) Butane and 2-methylpropane
(iv) Among the following the one having longest chain is
(a) neo-pentane
(b) iso-pentane
(c) 2-methylpentane
(d) 2,2-dimethylbutane.
Answer:
(i) (d) All of these
(ii) (a) four carbon atoms
(iii) (d) Butane and 2-methylpropane
(iv) (c) 2-methylpentane
Chapter – 5 Periodic classification of elements
- Read the following carefully and answer the questions.
Atoms of eight elements A, B, C, D, E, F, G and H have the same number of shells but different number of electrons in their outermost shell. It was found that elements A and G combine to form an ionic compound. This compound is added in a small amount to almost all vegetable dishes during cooking. Oxides of elements A and B are basic in nature while those of E and F are acidic. The oxide of D is almost neutral. Based on the above information answer the following questions:
(1) To which group or period of the Periodic Table do the listed elements belong?
(2) What would be the nature of compound formed by a combination of elements B and F?
(3) Which two of these elements could definitely be metals?
(4) If the number of electrons in the outermost shell of elements C and G be 3 and 7 respectively, write the formula of the compound formed by the combination of C and G.
Answer:
- A and B belong to group 1 and 2 because they form basic oxides. C belongs to group 13 as it has 3 valence electrons.
D belongs to group 14 as it forms almost neutral oxide.
E and F belong to group 15 and 16 as they form acidic oxides, G belongs to group 17 as it has 7 valence electrons and
H belongs to group 18.
They belong to 3rd period of the Periodic Table because AG is NaCl, added in a small amount to almost all vegetable dishes during cooking and Na and Cl belong to 3rd period.
- Ionic compounds will be formed because ‘B’ is metal and ‘F’ is non-metal. ‘B’ can lose two electrons and ‘F’ can gain two electrons.
- A and B are definitely metals as they form basic oxides.
- CG3 is the formula of the compound formed by combination of C and G.
Q. Read the following and answer the questions.
Today, 118 elements are known, the first 94 of which occur in nature. Of the 94 natural elements, eighty are stable.The periodic table is a graphic description of the periodic law, which states that the properties and atomic structures of the chemical elements are a periodic function of their atomic number. Elements are placed in the periodic table by their electron configurations, which exhibit periodic recurrences that explain the trends of properties across the periodic table. As we go across a period from left to right, we add a proton to the nucleus and an electron to the valence shell with each successive element. As we go down the elements in a group, the number of electrons in the valence shell remains constant, but the principal quantum number increases by one each time. An understanding of the electronic structure of the elements allows us to examine some of the properties that govern their chemical behavior. These properties vary periodically as the electronic structure of the elements changes.They are
(1) size (radius) of atoms and ions,
(2) ionization energies, and
(3) electron affinities.
- Which of the following set of elements is written in order of their increasing metallic character?
(a) Na, Li, K
(b) C, O, N
(c) Mg, Al, Si
(d) Be, Mg, Ca
- What happens to tendency to gain electron in a period?
(a) Increases,
(b) Decreases,
(c) Remaining same,
(d) First increases then decreases.
- Which of the following elements would lose an electron easily?
(a) Mg
(b) Na
(c) K
(d) Ca
4. Atomic size decreases from left to right in a period because
(a) Effective nuclear charge increases
(b) Number of shells remains the same
(c) Force of attraction between the nucleus and valence electrons increases
(d) All of these
Answer:
1 (d)
2 (d)
3 (c)
4 (d)
Chapter – 8 How do organisms reproduce?
- Read the paragraph and answer the questions.
The growing size of the human population is a cause of concern for all people. The rate of birth and death in a given population will determine its size. Reproduction is the process by which organisms increase their population. The process of sexual maturation for reproduction is gradual and takes place while general body growth is still going on. Some degree of sexual maturation does not necessarily mean that the mind or body is ready for sexual acts or for having and bringing up children. Various contraceptive devices are being used by human beings to control the size of population.
1) What are common signs of sexual maturation in boys
- a) Broadening of shoulders
- b) Development of mammary glands
- c) Broadening of waist
- d) High pitch of voice
2) Common sign of sexual maturation in girls is
- a) Low pitch voice
- b) Appearance of moustaches and beard
- c) Development of mammary glands
- d) Broadening of shoulders
3) Which contraceptive method changes the hormonal balance of the body
- a) Condoms
- b) Diaphragms
- c) Oral pills
- d) Both a) and b)
4) What should be maintained for healthy society
- a) Rate of birth and death rate
- b) Male and female sex ratio
- c) Child sex ratio
- d) None of these
Answer:
1) a) Broadening of shoulder
2) c) Development of mammary glands
3) c) Oral pills
4) b) Male and female sex ratio
Q. Read the following and answer the questions:
Preeti is very fond of gardening. She has different flowering plants in her garden. One day few naughty children entered her garden and plucked many leaves of Bryophyllum plant and threw them here and there in the garden. After few days, Preeti observed that new Bryophyllum plants were coming out from the leaves which fell on the ground.
- What does the incidence sited in the paragraph indicate?
(a) Bryophyllum leaves have special buds that germinate to give rise to new plant.
(b) Bryophyllum an propagate vegetatively through leaves.
(c) Bryophyllum is a flowering plant that reproduces only asexually
(d) Both (a) and (b).
- Which of the following plants can propagate vegetatively through leaves like Bryophyllum?
(a) Guava
(b) Begonia
(c) Ginger
(d) Mint
- Do you think any other vegetative part of Bryophyllum can help in propagation? If yes, then which part?
(a) Roots
(b) Stems
(c) Flowers
(d) Fruits
- Which of the following plant is artificially propagated (vegetatively) by stem cuttings in horticultural practices?
(a) Potato
(b) Snake plant
(c) Rose
(d) Water hyacinth
Answer:
- (d)
- (b)
- (b)
- (c)
Chapter – 9 Heredity and Evolution
- Read the following and answer questions from (i) to (iv)
Pea plants can have smooth seeds or wrinkled seeds. One of the phenotypes is completely dominant over the other. A farmer decides to pollinate one flower of a plant with smooth seeds using pollen from plant with wrinkled seeds. The resulting pea pod has all smooth seeds.
1) Which of the following conclusions can be drawn?
(i) The allele for smooth seeds is dominated over that of wrinkled seeds.
(ii) The plant with smooth seeds is heterozygous.
(iii) The plant with wrinkled seeds is homozygous.
(a) 1 only
(b) 1 and 2 only
(c)1 and 3 only
(d) 1, 2 and 3
2) Which of the following crosses will give smooth and wrinkled seeds in same proportion?
(a) RR X rr
(b) Rr X rr
(c) RRX Rr
(d) rr X rr
3) On crossing of two heterozygous smooth seeded plants (Rr), a total of 1000 plants were obtained in F1 generation. What will be the respective number of smooth and wrinkled seeds obtained in F1 generation
(a) 750, 250
(b) 500, 500
(c) 800, 200
(d) 950, 50
4)The characters which appear in the first filial generation are called
(a) recessive characters
(b) dominant characters
(c) lethal character
(d) non-mendelian characters.
Answer:
- (c) 1 and 3 only
- (b) Rr x rr
- (a) 750, 250
- (b) dominant characters
Q. Read the passage and answer the given five questions
Pea plant is also tiny, easy to grow, and produces a big number of offspring. Pea plants can have tall plants or dwarf plants. One of the phenotypes is completely dominant over the other. A farmer decides to pollinate one flower of a tall plant with using pollen from plant with dwarf plant. The resulting pea pod has all tall plants.
- Which of the following conclusions can be drawn?
(i) The allele for tallness is dominated over that of dwarfness.
(ii) The plant with tallness is heterozygous.
(iii) The plant with dwarfness is homozygous.
- (i) only
- (i) and (ii) only
- (i) and (iii) only
- (i), (ii) and (iii).
- Which of the following crosses will give tall and dwarf plants in same proportion
(a) TT x tt
(b) Tt x tt
(c) TT x Tt
(d) tt x tt
- Which of the following cross can be used to determine the genotype of a plant with dominant phenotype?
(a) TTXTT
(b) Tt x Tt
(c)Tt x TT
(d)Tt x tt
- State the ratio of tall plant to dwarf plants in F2 genertion. A. 2:2,
B.1:3 C.3:1, D.4:0
Answer:
- D. (i), (ii) and (iii).
- B. Tt x tt
- D. Ttxtt
- C. 3:1
Chapter – 12 Electricity
- Read the paragraph and answer the questions.
Electrical resistivities of some substances, at 20°C are given below in the table. Study the table and answer the given questions.
- Which is a better conductor of electric current ?
(A) Silver
(B) Copper
(C) Tungsten
(D) Mercury
- Which element will be used for electrical transmission lines ?
(A) Iron
(B) Copper
(C) Tungsten
(D) mercury U
- Nichrome is used in the heating elements of electric heating device because:
(A) It has high resistivity
(B) It does not oxidise readily at high temperature
(C) Both of the above
(D) None of the above U
- Series arrangement is not used for domestic circuits because:
(A) Current drawn is less
(B) Current drawn is more
(C) Neither of the above
(D) Both of the above U
Answer.
- (A)
- (B)
- (C)
- (A)
Q. Read the paragraph and answer the questions.
In the given circuit, three identical bulbs B1, B2 and B3 are connected in parallel with a battery of 4.5 V. Study the diagram and answer the questions given below :
- What will happen to the other two bulbs if the bulb B3 gets fused ?
(A) They will also stop glowing.
(B) Other bulbs will glow with same brightness.
(C) They will glow with low brightness.
(D) They glow with more brightness.
2.If the wattage of each bulb is 1.5 W, how much readings will the ammeter A show when all the three bulbs glow simultaneously?
(A) 1.0 A
(B) 2 A
(C) 1.5 A
(D) None of the above
- Find the total resistance of the circuit.
(A) 1.0 Ω
(B) 4.5 Ω
(C) 1.5 Ω
(D) 2.0 Ω
- How many resistors of 88 Ω are connected in parallel to carry 10 A current on a220 V line ?
(A) 2 resistors
(B) 1 resistors
(C) 3 resistors
(D) 4 resistors
Answer:
- (B)
- (A)
- (B)
- (D)
Chapter – 13 Magnetic effects of current
- Read the paragraph and answer the questions.
An electric motor is an electrical machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor’s magnetic field and electric current in a wire winding to generate force in the form of torque applied on the motor’s shaft.
1) Electric motor is used in
(a) electric fans
(b) refrigerators
(c) mixers
(d) all of the above
2) In Electric motor magnetic field is produced by
(a) Permanent magnet
(b) Electro magnet
(c) both (a) and (b)
(d) none of the above.
3) Direction of magnetic force on a current carrying conductor placed in magnetic field is given by
(a) Fleming’s left hand rule
(b) Fleming’s right hand rule
(c ) Right hand palm rule
(d) none of the above
4) Moving part of an electric motor is called (a)brushes
(b) shaft
(c) split ring
(d) slip ring
Answer:
- (a)
- (c)
- (a)
- (b)
Most important Topics for class 10 science board exam 2024
| Chapters | Important Topics |
| Chemical Reactions and Equations |
|
| Acids, Bases and Salts |
|
| Metals and Non-Metals |
|
| Carbon and its Compounds |
|
| Periodic Classification of Elements |
|
| Life Processes |
|
| Control and Coordination |
|
| How Do Organisms Reproduce? |
|
| Heredity and Evolution |
|
| Light- Reflection and Refraction |
|
| Human Eye and the Colourful World
|
|
| Magnetic Effects of Electric Current |
|
| Sources of Energy
|
|
| Our Environment |
|
| Management of Natural Resources |
|
Most important Topics for class 10 science board exam 2024 from Syllabus
| Unit No. | Unit | Marks |
| I | Chemical Substances-Nature and Behavior | 25 |
| II | World of Living | 25 |
| III | Natural Phenomena | 12 |
| IV | Effect of Current | 13 |
| V | Natural Resouces | 05 |
| Internal Assessment | 20 | |
| Grand Total | 100 |
CBSE Class 10 Science Important Topics: Preparation Tips
- Thoroughly read the NCERT science books. Typically, these texts are used as the basis for the question papers in the CBSE 10th board exams.
- As questions in exams are frequently repeated, always practise last year’s CBSE Class 10th Board question papers.
- After completing the curriculum, keep reviewing each topic frequently to ensure that students can remember it the day of the exam.
- One day or a few hours prior to the exam, avoid learning any new material. This will only lead to uncertainty and unneeded tension.
- Make digital or paper notes of crucial information to help you remember it more quickly. Use various study aids, such as flashcards, diagrams, underlining key passages in books, joining study groups, etc.
- Avoid studying for long periods of time at a time and take breaks as needed.
- Stay hydrated, consume wholesome foods, and steer clear of junk food.












 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...
 CUET PG Crash Course 2026: Subject-Wise ...
CUET PG Crash Course 2026: Subject-Wise ...






