The Chemistry subject becomes quite confusing and hard to memorize due to tons of equations and chemical reactions. So, memorizing all the equations and formulas seems tedious and highly time-consuming. With just 2 months left for the board exam, it is important to focus on the concepts from which questions are most likely asked in the board exam. This requires a deep understanding of the exam paper formulation and past paper analysis. You do not have to go through all of this, as we have toiled for your board exam’s sake and prepared the expected paper.
The expected Chemistry class 12 board question paper contains all the important questions that can be asked in the upcoming board exams. So, students going to take the board exam this year must solve the questions given in the expected board paper of the chemistry subject and match their answers with the standard solutions provided. In this way, they will not only gain confidence but will also increase their board marks.
Chemistry Class 12 Board Question Paper 2024
The CBSE Chemistry Examination is going to take place on 27th February 2024. So it is a very crucial time to maximize our score in the Class 12 Chemistry Exam. Students must practice the Model question papers for Chemistry as much as they can, it will beneficiary to face the questions in the Examination hall.
For the benefit of the class 12 candidates, Our Adda247 Expert faculty prepare the Class 12 Chemistry Expected Exam paper 2024 which is based on the actual CBSE Class 12 Exam pattern. Candidates who are going to take class 12 board exam this year must carefully go through the questions and solutions of the Class 12 Chemistry Expected exam paper 2024.
Chemistry Class 12 Board Exam Question Paper Revision
Chemistry Class 12 Board Question Paper 2024- Expected
Like the actual CBSE Class 12 Chemistry sample paper, Adda247’s Class 12 Chemistry Expected Test paper has 5 sections with 35 questions for 70 marks. Check out the marks distribution of the Class 12 Chemistry Expected Test paper given below;
1. Section A consists of 18 Questions carrying 1 mark each [Multiple Choice Questions]
2. Section B consists of 7 questions carrying 2 marks each [Very Short Answer Questions]
3. Section C consists of 5 questions carrying 3 marks each [Short Answer Questions]
4. Section D consists of 2 Questions carrying 4 marks each [Case Based Questions]
5. Section E consists of 2 Questions carrying 5 marks each [Long Answer Questions]
Also Check: Class 12 Chemistry Previous Year Question Papers With Solutions
Class 12 Chemistry Expected Test Paper Section A
SECTION A
The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.
Q1. The property which is not characteristic of transition metals is
(a) Variable oxidation states.
(b) Tendency to form complexes.
(c) Formation of coloured compounds.
(d) Natural radioactivity.
Q2. Which of the following is incorrect for KMnO4 to be used as an oxidising agent?
(a) HCl cannot be used because some KMnO4 is consumed in the reaction.
(b) Nitric acid is not used for the above purpose because it itself acts as a self-oxidising agent and will react with the reducing agent.
(c) The equivalent weight of KMnO4 in basic medium is 158.
(d) The number of electrons involved in oxidation of KMnO4 in acidic medium is 3.
Q3. Which one of the amino acids can be synthesised in the body?
(a) Alanine
(b) Lysine
(c) Valine
(d) Histidine
Q4. Which of the following is not true about amino acids?
(a) They are constituents of all proteins
(b) Alanine having one amino and one carboxylic group
(c) Most naturally occurring amino acids have D-configuration
(d) Glycine is the only naturally occurring amino acid which is optically inactive.
Q5. Alkyl halides are immiscible in water though they are polar because
(a) They react with water to give alcohols
(b) They cannot form hydrogen bonds with water
(c) C -X bond cannot be broken easily
(d) They are stable compounds and are not reactive
Q6. Which of the following compounds will have highest melting point?
(a) Chlorobenzene
(b) o-Dichlorobenzene
(c) m-Dichlorobenzene
(d) p-Dichlorobenzene
Q7. A zero-order reaction A → Products has a rate constant 10-2 mole L-1s-1 . If a process is started with 10 moles of A in a one litre vessel, the number of moles of reactant after 10 minutes will be
(a) 10
(b) 5
(c) 6
(d) 4.
Q8. For which of the following reactions, the temperature coefficient is maximum?
(a) A → B : Ea = 50 kJ
(b) P → Q : Ea = 40 kj
(c) X → Y : Ea = 60kJ
(d) W → Z : Ea = 80kJ
Q9. Which of the following is a secondary cell?
(a) Leclanche cell
(b) Lead storage battery
(c) Concentration cell
(d) All of these
Q10. For a certain redox reaction, E° is positive. This means that
(a) ΔG° is positive, K is greater than 1
(b) ΔG° is positive, K is less than 1
(c) ΔG° is negative, K is greater than 1
(d) ΔG° is negative, K is less than 1
Q11. The formula of potassium dicyanobis (oxalato) nickelate (II) is
(a) K4[Ni(CN)(Ox)2]
(b) K3[Ni2 (CN)2 (Ox)2]
(c) K4[Ni(CN)2(Ox)2]
(d) K2[Ni(CN)2(Ox)2]
Q12. The name of [Co(NH3)3 (NO2)3] is
(a) Triammine trinitro-N-cobalt(III)
(b) Trinitrotriamminecobalt(II)
(c) Trinititrotriamjninecobalt (III) ion
(d) Trinitrotnamminecobatate (III).
Q13. Vapours of an alcohol X when passed over hot reduced copper, produce an alkene, the alcohol is
(a) Primary alcohol
(b) Secondary alcohol
(c) Tertiary alcohol
(d) Dihydric alcohol
Q14. Picric acid is a yellow coloured compound. Its chemical name is
(a) m-nitrobenzoic acid
(b) 2, 4, 6-trinitrophenol
(c) 2, 4, 6-tribromophenol
(d) p-nitrophenol
Q15. An aqueous NaOH solution is added to a mixture of benzaldehyde and formaldehyde to produce
(a) benzyl alcohol + sodium formate
(b) sodium benzoate + methanol
(c) benzyl alcohol + methanol
(d) sodium benzoate + sodium formate
Q16. As a result of Wolff-Kishner reduction, the following conversions can be made:
(a) Benzaldehyde into Benzyl alcohol
(b) Cyclohexanol into Cyclohexane
(c) Cyclohexanone into Cyclohexanol
(d) Benzophenone into Diphenylmethane.
Q17. Which of the following is the strongest base?
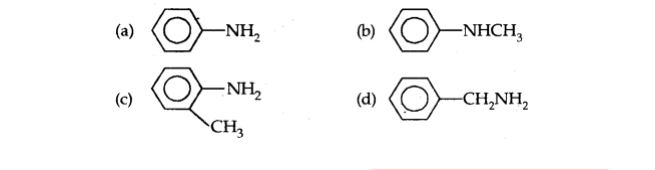
Important Questions for Class 12 Chemistry with answers
Class 12 Chemistry Expected Test Paper Section B
SECTION B
This section contains 7 questions with internal choice in two questions. The following questions are very short answer type and carry 2 marks each.
Q19. Given the standard electrode potentials,
K + /K = −2.93V, Ag+/Ag = 0.80V,
Hg2+/Hg = 0.79V
Mg2+/Mg = −2.37 V, Cr3+/Cr = − 0.74V
Arrange these metals in their increasing order of reducing power.
OR
The decomposition of NH3 on platinum surface is zero order reaction. What are the rates of production of N2 and H2 if k = 2.5 × 10−4 mol–1L s–1 ?
Q20. Write down the electronic configuration of:
(i) Co2+
(ii) Lu2+
(iii) Mn2+
(iv) Th4+
Q21. FeSO4 solution mixed with (NH4)2SO4 solution in 1:1 molar ratio gives the test of Fe2+ ion but CuSO4 solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of Cu2+ ion. Explain why.
Q22. Give the IUPAC names of the following compounds:
(i) CH3CH(Cl)CH(Br)CH3
(ii) CHF2CBrClF
(iii) ClCH2C≡CCH2Br
(iv) CH3C(p – ClC6H4)2CH(Br)CH3
Q23. Show how will you synthesise:
(i) 1-phenyl ethanol from a suitable alkene.
(ii) cyclohexyl methanol using an alkyl halide by an SN2 reaction.
Q24. What is meant by the following terms? Give an example of the reaction in each case.
(i) Cyanohydrin
(ii) Acetal
OR
What is meant by the following terms? Give an example of the reaction in each case.
(i) Ketal
(ii) Imine
Q25. Write the name of the cell which is generally used in transistors. Write the reaction taking place at anode and the cathode of this cell.
Class 12 Chemistry Expected Test Paper Section C
Section C
This section contains 5 questions with internal choice in two questions. The following questions are short answer types and carry 3 marks each.
Q26.A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of the solution is 1.2 g mL–1, then what shall be the molarity of the solution?
OR
State Henry’s law and mention some important applications?
Q27. Depict the galvanic cell in which the reaction Zn(s) + 2Ag+ (aq) → Zn2+(aq) + 2Ag(s) takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Q28. A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is (i) doubled (ii) reduced to half?
Q29. Explain briefly how the +2 state becomes more and more stable in the first half of the first-row transition elements with increasing atomic number?
Q30. Which one of the following has the highest dipole moment?
(i) CH2Cl2
(ii) CHCl3
(iii) CCl4
OR
What is meant by the hydroboration-oxidation reaction? Illustrate it with an example.
Class 12 Chemistry Expected Test Paper Section D
Section D
The following questions are case-based questions. Each question has an internal choice and carries 4 (1+1+2) marks each. Read the passage carefully and answer the questions that follow.
Q31. Read the passage given below and answer the following questions:
The sequence of bases along the DNA and RNA chain establishes its primary structure which controls the specific properties of the nucleic acid. An RNA molecule is usually a single chain of ribose-containing nucleotide. On the basis of X-ray analysis of DNA, J.D. Watson and F.H.C.crick (shared noble prize in 1962) proposed a three dimensional secondary structure for DNA. DNA molecule is a long and highly complex, spirally twisted, double helix, ladder like structure. The two polynucletide chains or strands are linked up by hydrogen bonding between
the nitrogeneous base molecules of their nucleotide monomers. Adenine (purine) always links with thymine (pyrimidine) with the help of two hydrogen bonds and guanine (purine) with cytosine (pyrimidine) with the help of three hydrogen bonds. Hence, the two strands extend in opposite directions, i.e., are antiparallel and complimentary.
In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct anwer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but assertion is correct statement.
(I) Assertion: DNA molecules and RNA molecules are found in the nucleus of a cell.
Reason: there are two types of nitrogenous bases, purines and pyrimidines. Adenine (A) and guanine (G) are substituted purines, cytosine (C), thymine (T) and uracil (U) are substituted pyrimidines.
(II) Assertion: In both DNA and RNA, heterocyclic base and phosphate ester linkage are at C-1’ and C5’respectively of the sugar molecule.
Reason: Nucleotides and nucleosides mainly differ from each other in presence of phosphate units.
OR
Assertion: The backbone of RNA molecule is a linear chain consisting of an alternating unit of a heterocyclic base, D-ribose and a phosphate.
Reason: The segment of DNA which acts as the instruction manual for the synthesis of protein is ribose. (III) Define nucleotide and nucleoside
Q32. Read the passage given below and answer the following questions:
Both alcohols and phenols are acidic in nature, but phenols are more acidic than alcohols. Acidic strength of alcohols mainly depends upon the inductive effect. Acidic strength of phenols depends upon a combination of both inductive effect and resonance effects of the substituent and its position on the benzene ring. Electron withdrawing group increases the acidic strength of phenols whereas electron donating groups decreases the acidic strength of phenols. Phenol is a weaker acid than carboxylic acid.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Phenols are highly acidic as compare to alcohols due to
(a) The higher molecular mass of phenols
(b) The stronger hydrogen bond in phenols
(c) Alkoxide ion is a strong conjugate base
(d) Phenoxide ion is resonance stabilized.
(ii) The correct order of acidic strength among the following is:
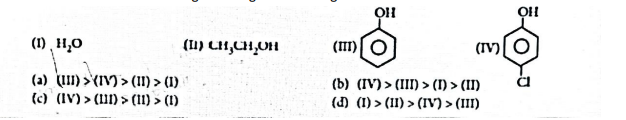
OR
The correct decreasing order of pKa value is
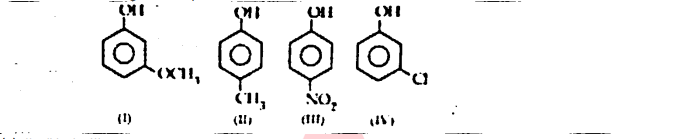
(a) II > IV > I > III
(b) IV > II > III > I
(c) III > II > IV > I
(d) IV > I > II > III
(iii) Out of o-nitrophenol and o-cresol which is more acidic?
Class 12 Chemistry Expected Test Paper Section E
SECTION E
The following questions are long answer type and carry 5 marks each. Two questions have an internal choice.
Q33. Define the term solution. How many types of solutions are formed? Write briefly about each type with an example.
OR
(i) Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
(ii) What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
Q34. (i) An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene. Write equations for the reactions involved.
(ii) Give one chemical test to distinguish between the following pairs of compounds.
(a) Aniline and benzylamine
(b) Aniline and N-methylaniline.
OR
Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Q35. (i) Account for the following:
(a) Aniline does not undergo Friedel-Crafts reaction.
(b) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(c) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
(ii) What happens when D-glucose is treated with the following reagents?
(a) HI
(b) Bromine water
(c) HNO3
Chemistry Class 12 Board Question Paper 2024- With Solutions
We have also provided you with detailed solutions for Class 12 Chemistry Expected Test paper 2024. So, after solving the Class 12 Chemistry Expected Test paper 2024, students can analyze their scores. We have provided two expected test papers on the latest exam pattern so that students can cover the most likely questions that can be encountered in the exam. The solutions of these questions have also been mentioned to help students in their preparation.
| Class 12 Chemistry Expected Test Paper With Solutions Download |
| Chemistry Class 12 Board Expected Question Paper 2024 With Solutions |
Chemistry Class 12 Board Sample Paper 2024 With Solutions
The detailed solution video of the Chemistry Class 12 Board official sample paper 2024 is given below.











 CBSE Admit Card 2026 for Private & R...
CBSE Admit Card 2026 for Private & R...
 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...






