Take a moment to think about your present state of breath. Yes, right now, while you’re reading this. Doesn’t it feel like nothing? Nothing but an invisible, weightless vapor. That nothingness is air, and we are all aware of how important air is to our survival. But what exactly is air? The atmosphere, often known as air, is a mixture of different gases and other components. In this article, we will learn about various Air Components of air and their brief explanations.
Components of air
Air is the most important component of our existence. Air is all around us, although it is invisible to the naked sight. It is a gas combination that is often known as the atmosphere. To put it simply, it is a gas mixture that occupies space that is not occupied by solid or liquid stuff. The first thing that comes to mind when we think of air is oxygen. Aside from oxygen, numerous additional airborne molecules are required for life on Earth to survive. The majority of air’s constituents are nitrogen and oxygen, with a liberal pinch of argon, carbon dioxide, and a sprinkling of other trace gases.
What is the Primary Component of Air?
The primary component of air is nitrogen (N₂), which makes up about 78% of the Earth’s atmosphere by volume. The next most abundant component is oxygen (O₂), accounting for about 21%. The remaining 1% includes other gases like argon, carbon dioxide, neon, helium, and trace amounts of other gases.
Main Components of Air
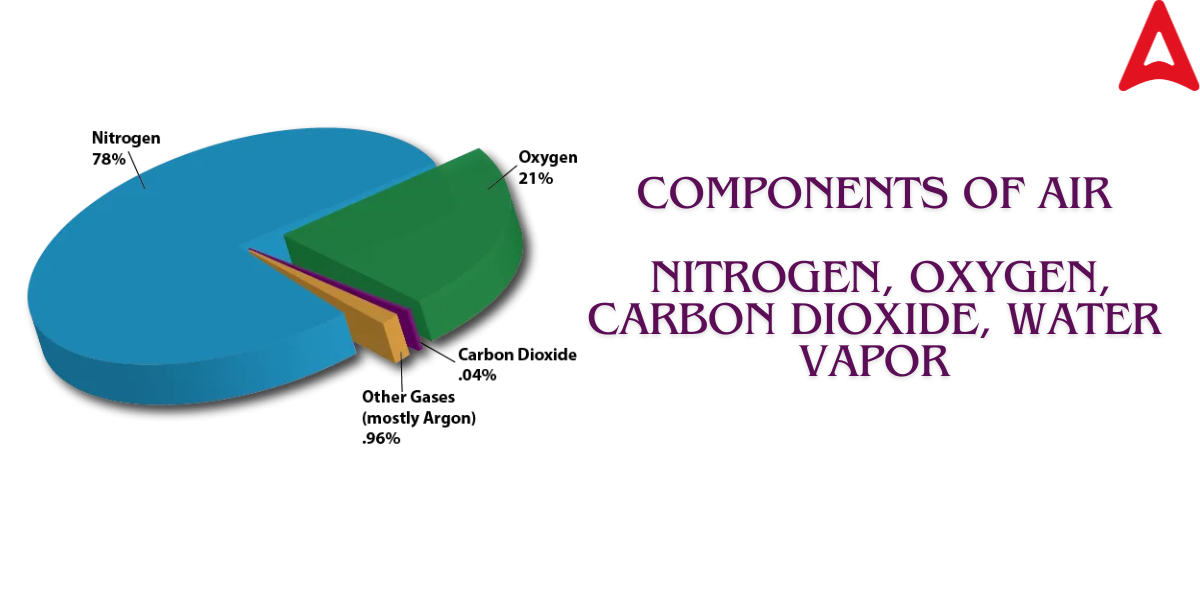
The main components of air are: Nitrogen (N₂) – Approximately 78%, Oxygen (O₂) – Approximately 21%, Argon (Ar) – About 0.93%, Carbon Dioxide (CO₂) – About 0.04%, Water Vapor (H₂O) – Varies from 0% to 4%, depending on humidity, Other trace gases – Includes neon, helium, methane, krypton, hydrogen, and ozone in very small amounts.
These components form the Earth’s atmosphere and are essential for life and various natural processes.
Components of Air and Their Percentages
Air is composed of 78.09% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and trace amounts of other gases. Water vapor and dust particles are also constituents of air in various proportions.
The composition of air varies from place to location and is not consistent. For example, in polluted places, carbon dioxide emissions are particularly high, therefore they have a larger percentage of carbon. Check the below chart that shows the percentage of various components of Air.
Air Diagram
Air Diagram is given below. check all component air with their percentages.
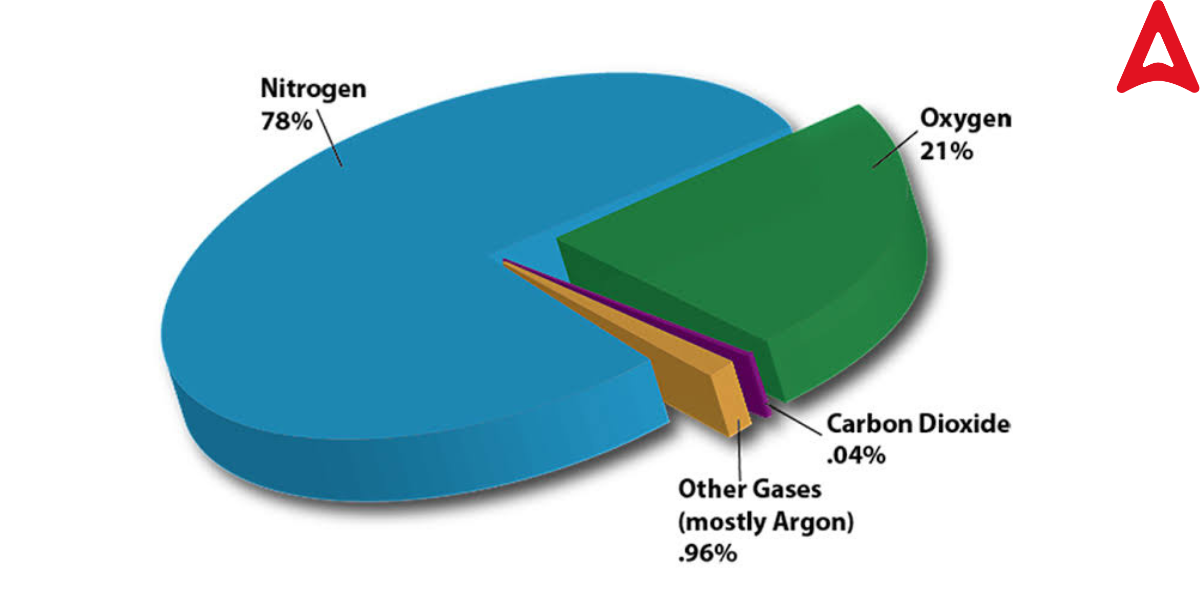
Constituents of Air
Air is a mixture of various gases, with the major constituents being:
Nitrogen (N₂) – About 78% of the air.
Oxygen (O₂) – Approximately 21%.
Argon (Ar) – About 0.93%.
Carbon Dioxide (CO₂) – Around 0.04%.
Other Gases – Trace amounts of gases like neon, helium, methane, krypton, and hydrogen.
Water Vapor (H₂O) – Varies depending on humidity and location, typically 0-4%.
These components make up the air we breathe and are essential for life and various natural processes on Earth.
Components of Air in Brief
Air was once assumed to be a pure substance, but it was later discovered to be a mixture of several gases. The components of air are numerous. The following are the main components of air:
Nitrogen: The Silent Majority
Nitrogen gas occupies the majority of are among all the components of air. If air was a class, nitrogen would be that quiet kid who doesn’t say much but makes up the majority of the group. It accounts for about 78% of the air we breathe.
Our quiet kid, Nitrogen, plays a crucial role by diluting oxygen and preventing rapid burning or combustion. Picture this – you’re roasting marshmallows over a campfire, and suddenly the entire camp is ablaze. That’s what would happen without our silent majority.
Oxygen: The Breath of Life
Oxygen, the life of the party, makes up about 21% of air. Every living organism’s survival hinges on it. We inhale oxygen, it teams up with glucose in our body to produce energy, and then we exhale carbon dioxide. It’s a neat, essential process that keeps us alive.
Our bodies are like machines, and oxygen is the fuel that powers us. It boosts cellular growth, aids in the healing process, and powers up the human brain. Oxygen supports combustion. It’s kind of like adding fuel to the fire. Without oxygen, candles won’t burn, and engines won’t run. It’s also involved in different chemical reactions to produce substances like water and carbon dioxide.
Argon: The Noble Participant
Argon is the cool kid among the all components of air., making up about 1% of the air. Argon’s great at keeping to itself. The noble gas is incredibly stable and doesn’t react with other elements easily—which is why it’s called “noble.” It’s colorless, odorless, tasteless, and belongs to a group of elements called “Noble Gases.” If Argon was a person, it would be the one sipping a cocktail in the corner of a party, minding its own business. Despite its aloofness, argon has some important uses. It’s used in light bulbs, neon lights, welding, and even protects historical documents by creating an unreactive environment.
Carbon Dioxide: More Than Just Exhalation
Carbon dioxide has a concentration of around 0.04% among all components of air. It has the atomic number 6 and the chemical symbol CO2. Carbon dioxide is mostly created by plant and animal respiration or by the combustion of fuel.
In an interesting twist of events, the carbon dioxide that we exhale during respiration is used by plants during photosynthesis. And what do they release? Oxygen, which we breathe in! This is nature’s brilliant recycling program at its finest.
Carbon Dioxide’s Part in Climate Change
Here’s the thing, though. Carbon dioxide traps heat and too much of it can cause the Earth’s temperature to rise, leading to global warming. And who’s adding to this soup of carbon dioxide? We are, through activities like deforestation, burning fossil fuels like coal and oil, and manufacturing processes in industries.
Trace Gases: The Minor Yet Vital Components
Trace gases are gases that are present at very minute levels in the atmosphere. In the recipe that is air, trace gases are the seasonings, present in tiny amounts, but vital nonetheless. These include neon, helium, methane, and others.
Think about the cheerful red and orange glow of neon lights or the fun in a helium-filled balloon. That’s the contribution of these trace gases. Methane though, is a double-edged sword. It helps generate electricity and heat, but is also a potent greenhouse gas! While these gases contribute to daily life, an increase due to human activity can disrupt air quality and harm the environment.
Particulates and Pollutants: The Unwelcomed Guests
Air, like a house, can have unwelcome guests – particulates and pollutants. These include dust, pollen, smoke, and chemical fumes. These uninvited guests decrease air quality and create smog. It’s like throwing a house party you didn’t plan, and now your house is a mess. Pollutants can lead to respiratory problems and damage the environment by causing events like acid rain. That’s one house party gone horribly wrong.
Water vapor and Dust particles
Water vapor is also considered as one of the components of air in various proportions. When water in bodies of water evaporates owing to heat, it rises and mixes into the atmosphere. The humidity level can be used to determine the presence of water vapor in the air. The molar mass of dry air or air with little or no water vapor is 28.97g/mol.
Smoke and dust particles
Smoke and dust particles make up less than 1% of the Earth’s atmosphere. Dust consists of small solid particles. Aeolian dust is a type of atmospheric dust. These particles are composed of particles emitted by volcanic eruptions, pollution, or soil. These dust particles contain pollens, human and animal hair, and soot particles in trace amounts. Smoke, on the other hand, is present in our atmosphere as a result of the combustion of fuels. Its occupancy fluctuates depending on location.
Importance of Air
There’s a reason why they say, “air is life.” Animals need it to breathe, plants rely on it for photosynthesis, and it’s a crucial part of Earth’s atmosphere that protects us from harmful radiation. Imagine you’re baking a cake. You need flour, sugar, and eggs, right? Air’s pretty much like that. Its components work together to facilitate life on Earth in varied ways.
Air is a beautifully complex mixture of gases, each serving a specific purpose. Like in a band, every gas plays its part in the mesmerizing symphony of life and the environment.
The Balance of These Components of Air in Maintaining Life. When Earth’s atmospheric composition plays in harmony, life thrives. Disturb this balance, and we waltz toward harmful environmental issues.








 Chemistry Investigatory Project Class 12...
Chemistry Investigatory Project Class 12...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...
 CUET Chemistry Syllabus 2026, Download O...
CUET Chemistry Syllabus 2026, Download O...














