Table of Contents
Coulomb’s Law
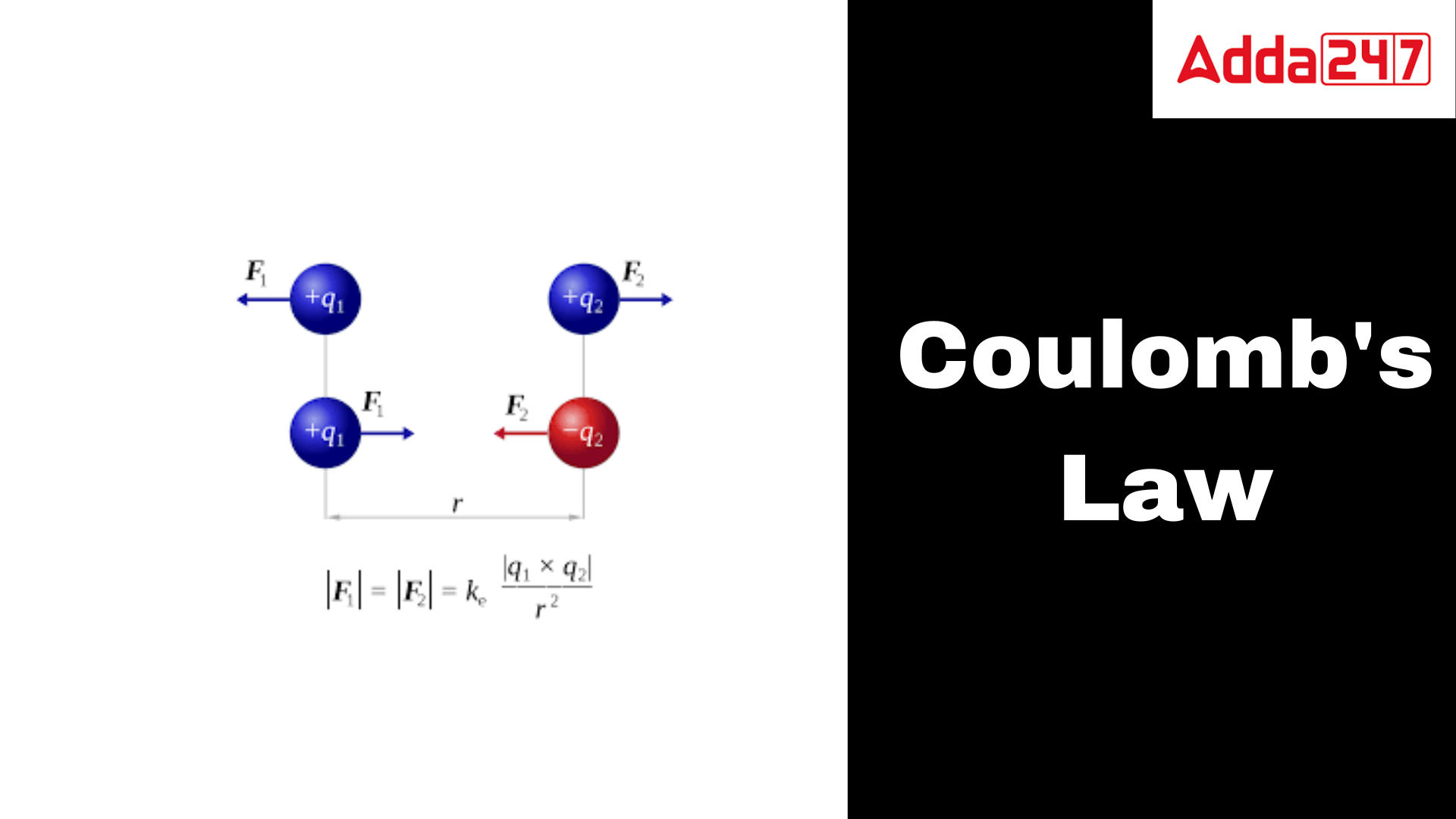
Coulomb’s Law: According to this law, the electrical force between two charged objects is equal to the product of such charged particles and inversely proportional to the square of their distance. Attractive or repulsive force acts. Electrostatic force describes it. It measures the force between two charged particles. French physicist Charles-Augustin de Coulomb introduced this idea in 1785. Straight-line force. The force is repulsive if the charged object has the same sign and attractive if it has the opposite sign.
Coulomb’s Law Definition
The force of attraction or repulsion between two electrically charged particles is defined by Coulomb’s law, a physical principle. According to the rule, the strength of the force is inversely proportional to the square of the distance between the charges and directly proportional to the product of the charges.
Coulomb’s law can be formulated mathematically as follows:
Where: F is the force between the two charges and F = k * q1 * q2 / d2
The Coulomb constant, or k, has a value of 9 * 109 N * m2 / C2 and is a constant.
The two particles’ charges are q1 and q2, respectively.
d represents the separation of the two particles.
If the two charges have opposing signs, the force between them is attracting; if they have the same sign, it is repulsive. If the charges are greater, the force is stronger; if the charges are farther apart, the force is weaker.The fundamental physical principle known as Coulomb’s law is crucial to the study of electromagnetic, chemistry, and biology, among other branches of science.
Coulomb’s Law Formula
As we are all aware, coulomb’s law states that the product of two charged objects and the distance between them are directly proportional to each other. In mathematics, it can be expressed as:
F ∝ q1q2/d²
F = 1/4π€° x q1q2/d²
Where,
After the proportion is subtracted, we have a proportionality constant of 1/4°, which equals the value of 9109 N-m2/C2.
These two charged bodies are q1 and q2.
The distance between the two bodies is squared as d2.
Units, Dimensions, and Value of Coulomb’s Law
€° = 1/4πF x q1q2/d²
As SI Unit of charge is coulomb(C)
Therefore,
Unit of €° = 1CC/ Nm² = C²N^-1m^-2
Dimensions of €° = [M^-1L^-3T^4A^2]
Value of €°= 8.85 x 10^-12 C²N^-1m^-2
Coulomb’s Law Vector form
Scalar and vector quantities are the two different forms of quantities. While a vector quantity determines both the direction and the magnitude, a scalar variable just decides the magnitude. Force is a vector quantity that has both magnitude and direction, as is common knowledge. Thus, Coulomb’s law is expressed as a vector.
Suppose there are two charges, q1 and q2, respectively. Since both charges have the same sign, the force operating between them will be repulsive. Let F12 be the force on the q1 charge caused by Q2 and F21 be the force on the q2 charge caused by Q1. The force acting on q2 as a result of q1 is opposite to and equal to the force on q2 as a result of q1. The third law of motion of Newton is thus observed by Coulomb’s law.
F12 = -F21
Therefore, F12 = 1/4° x q1q2/d2 = -F21 represents the force acting in vector form
Coulomb’s law Example and Applications
Here are some examples of how Coulomb’s law is used in daily life:
- Magnets are drawn to one another because their magnetic poles are in opposition to one another. Coulomb’s law governs the force of attraction between two magnets.
- When a balloon is rubbed against your hair, it gets negatively charged due to the attraction between charged particles. If you bring the balloon close to another negatively charged object, the other thing will be repelled if it is likewise negatively charged. This is due to Coulomb’s law, which states that because the negatively charged balloon and the negatively charged item have the same sign of charge, they repel one another.
- Atomic stability: Protons and electrons, which are both positively and negatively charged, make up an atom. The electrostatic force, which holds the atom together, pulls the electrons towards the protons. Coulomb’s law establishes the magnitude of the electric force between electrons and protons.
- Electrostatic precipitation: This method for removing dust and other airborne particles from the atmosphere. By putting the particles through an electric field, the particles are charged. After that, a grounded plate attracts the charged particles, where they are subsequently gathered.
- Xerography: Xerography is a photocopying technique that imprints an image on paper using electrostatics. A paper is passed over a negatively charged drum, which allows part of the positive charge from the document to transfer to the drum. Then a powder that is negatively charged is sprinkled onto the drum. The parts of the drum that have been charged by the document are where the powder adheres. The powder is then melted and fused to the paper by heating the drum.
- Electrostatics are used by ink-jet printers to spray ink onto paper. Above the paper is a plate that is negatively charged. Ink droplets are positively charged after being passed via an electric field. The negatively charged plate attracts the charged droplets, which are then sprayed onto the paper there.
- In order to apply a coating to a surface, electrostatic painting is a technique. A charged mist of the coating is sprayed onto the surface. The mist’s charged particles are drawn to the surface and deposition occurs there.
Limitations of Coulomb’s law
The following are some of Coulomb’s law’s drawbacks:
- Coulomb’s law only applies to point charges, meaning that it assumes there is no spatial dimension to the charges. In fact, every charge has a limited size. This indicates that Coulomb’s law does not perfectly anticipate the force between two charges.
- The consequences of polarization are not taken into consideration: A neutral object will become electrically charged through the process of polarisation when an electric field is present. Polarization’s effects are not taken into consideration by Coulomb’s law. This means that if the items are polarised, the force between two charges may change slightly from what Coulomb’s law predicts.
- Coulomb’s law only applies to static charges and does not apply to moving charges. Magnetic fields are created by moving charges, and the force between moving charges is not just the sum of their electrostatic forces.
- Coulomb’s law is invalid in extremely powerful electric fields, thus those don’t apply. Since quantum mechanics controls how charges behave in these fields, Coulomb’s law is insufficient to adequately describe the force that exists between charges.
Exercise of Coulomb’s law Problems
Question 1: Two-point charges, q1 = +12 μC and q2 = 8μC are separated by a distance r = 20 cm. Calculate the magnitude of the electric force.
Answer: Here, q1 = +12 μC = 12 x 10^-6 C
q2 = 8μC = 8 x 10^-6C
r = 20 cm = 0.20m
According to Coulomb’s law, F = 1/4π€° x q1q2/d²
F = (9×10^9) (12 x 10^-6) (8 x 10^-6)/ (0.20)²
F = 25.92 N


 UP, MP, CBSE Board Result 2025 Live Upda...
UP, MP, CBSE Board Result 2025 Live Upda...
 CUET UG Exam Date Sheet 2025 @cuet.nta.n...
CUET UG Exam Date Sheet 2025 @cuet.nta.n...
 NTA NEET Cut Off 2025, Expected Cutoff f...
NTA NEET Cut Off 2025, Expected Cutoff f...










