Table of Contents
A covalent Bond is a class of Chemical bonding in which the chemical bond formed between two atoms consists of the mutual sharing of one or more pairs of electrons.
What is Covalent Bond Class 10
A Covalent Bond is formed when atoms of two nonmetal elements share an electron pair between them. In organic chemistry, a covalent bond is more frequent rather than Ionic bonds. Some nonmetals that show covalent bonds are Hydrogen (H2), Carbon (C), Methane (CH4), Water (H2O), and so on. In this article, we will go to learn about different types of covalent bonds, the properties of covalent bonds, and their formation. Let’s start learning.
Define Covalent Bond Class 10
Covalent Bond: A chemical bond between atoms that are created when one or more pairs of electrons, every two atoms exchange an equal amount of electrons or pairs of electrons made up of a covalent bond. When the difference between the electro-negativities of two atoms is too small for an electron transfer to take place to create ions, a covalent bond is formed.
When atoms share electrons, a permanent equilibrium of the attractive and repulsive forces between them is known as covalent bonding. These electron pairs are also known as bonding pairs or sharing pairs.
For example, oxygen (O2), chlorine (Cl2), phosphorus trichloride (PCl3), ethanol(CH3CH2OH ), Methane (CH4), Ozone (O3), hydrogen ( H2), water (H2O), hydrogen chloride (HCl)
Covalent Bond Examples
Here we provided some examples of their Structures.
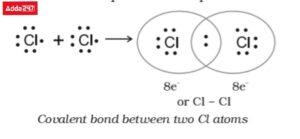
• Structure of Chlorine (Cl2)
In the chlorine atom, Cl2, two atoms share an electron pair. Covalent bonding is what causes this. Therefore, atoms are able to fill their valence shell and so achieve a noble gas configuration by sharing electrons through the formation of covalent bonds.
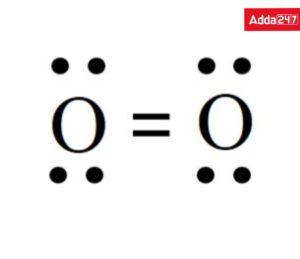
• Structure of Oxygen (O2)
Four dots and two sticks or lines surround each O, signifying an additional 4 electrons in the O2 double bond. As a result, each O has an octet and 8 total valence electrons surrounding it, making it stable.
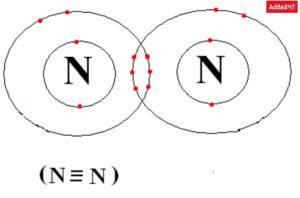
• Structure of Nitrogen (N2)
The N2 triple bond’s additional 6 electrons are represented by the two dots, three sticks, and three lines that encircle each N. In order to give each N an octet and make it stable, there are 8 total valence electrons surrounding it.
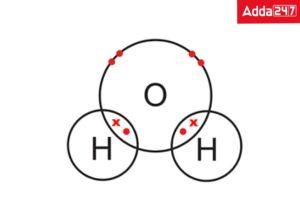
• Structure of Water ( H2O)
Hydrogen and oxygen share electrons within the water molecule. Each end of the V-shaped H2O molecule adopts a slightly different charge because oxygen and hydrogen draw the shared electrons from each other in different ways.
10 Examples of Covalent Bonds
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. Here are 10 examples of covalent compounds:
- Water (H2O): Two hydrogen atoms share electrons with one oxygen atom to form a water molecule.
- Methane (CH4): One carbon atom shares electrons with four hydrogen atoms to create methane.
- Ammonia (NH3): A nitrogen atom shares electrons with three hydrogen atoms in ammonia.
- Carbon dioxide (CO2): Two oxygen atoms share electrons with one carbon atom in carbon dioxide.
- Methanol (CH3OH): One carbon atom shares electrons with three hydrogen atoms and one oxygen atom in methanol.
- Hydrogen chloride (HCl): A hydrogen atom shares electrons with a chlorine atom in hydrogen chloride.
- Carbon tetrachloride (CCl4): One carbon atom shares electrons with four chlorine atoms in carbon tetrachloride.
- Oxygen gas (O2): Two oxygen atoms share electrons to form the oxygen molecule.
- Nitrogen gas (N2): Two nitrogen atoms share electrons to create the nitrogen molecule.
- Ethene (C2H4): Two carbon atoms share electrons with each other and with two hydrogen atoms each in the ethene molecule.
These examples illustrate various covalent compounds where atoms bond together by sharing electrons to achieve a stable, filled outer electron shell.
Covalent Bond Types
The Covalent bond is basically of three types. Such as
Single covalent bond
When an electron pair is shared equally between two atoms, a covalent single bond is formed between the two atoms. Single Covalent Bond is denoted by the symbol ‘-‘.
For example, a covalent bond is formed in the case of H2 molecule (H – H), Cl2 molecule (CI – CI), or HCI molecule (HCl).
Double covalent bond
When two electron pairs are shared equally between two atoms, a covalent double bond is formed between the two atoms. Double covalent bonds s denoted by the symbol ‘=’.
For example, the covalent double bond is formed in the case of the O2 molecule (0=0). Covalent triple bond: When two atoms share three electron-pairs
Triple covalent bond
A triple bond is a covalent bond between two atoms that involves six bonding electrons rather than the normal one or two-covalent bond. As results a covalent bonds with triple bond order, triple bonds are stronger than the corresponding single covalent bond or double covalent bond.
Triple Covalent Bond is denoted by ‘≡’ sign.
For example, a covalent triple bond is formed in the case of N2 molecule (N≡N) , carbon monoxide (C≡O)
Polar covalent bond
This kind of covalent bond occurs when joining atoms have different electronegativities, resulting in an unequal sharing of electrons. Atoms having more electronegative will attract electrons with greater force. Between the atoms. The common pair of electrons will consequently be nearer to that atom as a result.
As an instance, we consider molecules that hydrogen bond because of an imbalanced electrostatic potential. Here, the hydrogen atom interacts with fluorine, hydrogen, or oxygen, all of which are electronegative elements.
Nonpolar Covalent bond
If the sharing of electron pair equal distribution among the atoms, then, this type of covalent bond is created. There is no difference in the electronegativity of the two atoms. It formed Wherever the combining atoms have comparable electron affinities.
Gas molecules like hydrogen gas, nitrogen gas, and others contain nonpolar covalent bonds for example.
Covalent bond is Formed by Electrons
In most cases, covalent bonding occurs between nonmetals. Atoms that share electrons and have similar electronegativity are said to form covalent bonds. The kind of link that holds the atoms in a polyatomic ion together is covalent bonding. A covalent bond requires two electrons, one from each bonding atom.
Covalent bond- What is the octet rule?
Expect the few noble gases, all atoms have less than eight electrons in their valence shell. The octet rule is that those main-group elements typically bind in a way that each atom has eight electrons in its valence shell, giving it the same electrical configuration as a noble gas. The tendency of an element’s atom to acquire a stable configuration of eight electrons in its valence shell is known as the Octet rule. Specifically, carbon, nitrogen, oxygen, and halogens are shown octet rule.
Covalent bond- Lewis Dot Structure
The structure of a covalent compound is expressed by writing a pair of dots between the two atoms to form a covalent bond, indicating the electrons with dots, called the Lewis dot structure. Electrons are represented by a dot (.) or cross (x) symbol. Lewis point structure of furin molecule and hydrogen chloride molecule is shown on the side.
The Lewis dot structure of a molecule or ion can be written in the following steps.
Step 1: The total number of valency electrons present in the atoms must be calculated.
Step 2: If it’s an anion, how many units are negative charge present, add the number of electrons equal to the charge, and if it is a cation, subtract the number of electrons equal to the unit positive charge. Thus the total number of distributable electrons will be found.
Step 3: Draw a framework with the atom with the lowest electronegativity as the central atom. Note that H and F are usually in marginal positions.
Step 4: Place a pair of electrons between every two atoms to denote a single bond. The remaining electrons must be used for multiple bonds or lone pairs. It should be remembered that each atom should fill the octave.
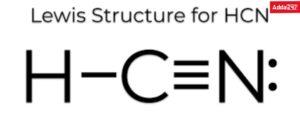
Example Lewis dot structure drawing of HCN molecule.
1. Total number of valence electrons of atoms in HCN molecule =1+4+5=10
2. Structure of compound HCN (Carbon less than Nitrogen electro-negative).
3. Write a pair of electrons between H and C and a pair of electrons between C and N and write the remaining electrons as lone pairs give.
4. In this structure, a doublet of hydrogen is filled but an octet of carbon and nitrogen is not filled. Therefore, multiple bonds between carbon and nitrogen are required. Carbon and nitrogen are octets filled if they have a triple bond between them. Thus, the Lewis dot structure of the compound is:
Covalent bond of Water Properties
when more than one element atoms share one or more electron pairs to the covalent bond between those atoms, the bonding ability of those elements is called covalency, and the elements participating in the formation of a covalent bond are called covalent compounds. Some Properties of Covalent Compounds are pointed below
1. Typically, covalent compounds only consist of a single molecule. At normal temperatures and pressures, these molecules primarily exist as gaseous, liquid, or soft-solid entities since the forces of mutual attraction between them are quite weak.
2. Because the intermolecular forces of attraction between the molecules of covalent compounds are so weak, it doesn’t take much energy to separate one molecule from another. As a result, the melting and boiling points of covalent compounds are typically lower than those of ionic compounds.
3. Covalent Bond compounds are mostly non-conductors. Molecules of covalent bond compounds do not dissociate into ions when dissolved or molten, so they do not transfer electricity when dissolved or molten.
4. Covalent compounds are soluble in organic solvents but insoluble in water.
5. Ionic bonds are more powerful than covalent bonds.
6. Covalent bonds are directional as a covalent bond is created by the overlapping of atomic orbitals which are half-filled and have distinct orientations.
Covalent Bond vs Ionic bond
| Points of Difference | Covalent Bond | Ionic Bond |
|---|---|---|
| Polarity | Low | High |
| Occurrence | Two non-metals | One metal and one non-metal |
| Formation | The sharing of pairs of electrons between atoms and other covalent bonds define covalent bonding, between two non-metallic atoms. | An ionic bond is created in a chemical molecule by the electrostatic attraction of ions with opposing charges between One metal and one non-metal |
| Shape | Definite shape | No definite shape |
| Melting Temperature | low | High |
| Conductor | Poor conductor | Good conductors of electricity when dissolved or melted |
| Electronegativity | The bigger an atom’s electronegativity, the more aggressively it will draw electrons from its bonds. The atom with the partial negative charge is the more electronegative atom because electrons in a polar covalent connection are moved toward it. | Ionic bonds forms between atoms with different electronegativity values from one another |
| Boiling point | Low | High |
| State | Liquid or gaseous | Solid |
| Examples | Methane (CH4),Nitrogen (N2), Hydro Chloric acid (HCl) | Sodium chloride (NaCl), Sulphuric Acid (H2SO4 ) |
Covalent Bond Important Questions for Class 10 and 11
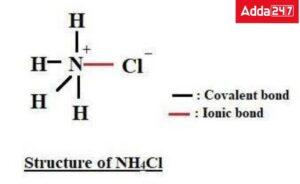
Q. Which of the following compounds has both an ionic and a covalent bond?
a. HCl
b. NaCl
c. NH4Cl
d. H2SO4
Answer: A covalent bond is present between N and HAtom and an ionic bond is present between NH4+ ion and Cl- ion.
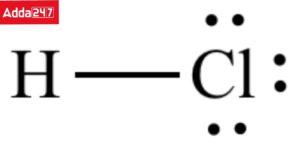
Q. What is the lewis dot structure of HCl?
Answer: Each of the chlorine and hydrogen atoms provides one electron to the common pair of electrons. The chlorine atom gains the octet of electrons whereas the hydrogen atom gains the duplet of electrons during the process.
Q. What is triple Covalent Bond?
Answer: A triple bond is a covalent bond between two atoms that involves six bonding electrons rather than the normal one or two-covalent bond. As results a covalent bonds with triple bond order, triple bonds are stronger than the corresponding single covalent bond or double covalent bond.
Triple Covalent Bond is denoted by ‘≡’ sign.
For example, a covalent triple bond is formed in the case of N2 molecule (N≡N), carbon monoxide (C≡O)
Q.What structure does a chlorine molecule have?
The structure of chlorine molecules is Covalent. In the chlorine atom, Cl2, two atoms share an electron pair. Covalent bonding is what causes this. Therefore, atoms are able to fill their valence shell and so achieve a noble gas configuration by sharing electrons through the formation of covalent bonds.
Define Covalent Bond in Hindi
सहसंयोजक बंधन
एक सहसंयोजक बंधन रासायनिक बंधन का एक वर्ग है जिसमें दो परमाणुओं के बीच बनने वाले रासायनिक बंधन में एक या एक से अधिक जोड़े इलेक्ट्रॉनों की पारस्परिक साझेदारी होती है। A सहसंयोजक बंधन तब बनता है जब दो अधातु तत्वों के परमाणु उनके बीच इलेक्ट्रॉन युग्म साझा करते हैं। कार्बनिक रसायन विज्ञान में, आयनिक बंधों के बजाय एक सहसंयोजक बंधन अधिक बार होता है। कुछ अधातुएँ जो सहसंयोजक बंध दिखाती हैं, वे हैं हाइड्रोजन (H2), कार्बन (C), मीथेन (CH4), जल (H2O), इत्यादि। इस लेख में, हम विभिन्न प्रकार के सहसंयोजक बंधों, सहसंयोजक बंधों के गुणों और उनके गठन के बारे में जानेंगे। आइए सीखना शुरू करें।
सहसंयोजक बंधन परिभाषा
सहसंयोजक बंधन: परमाणुओं के बीच एक रासायनिक बंधन जो तब बनता है जब इलेक्ट्रॉनों के एक या अधिक जोड़े, प्रत्येक दो परमाणु एक समान मात्रा में इलेक्ट्रॉनों या एक सहसंयोजक बंधन से बने इलेक्ट्रॉनों के जोड़े का आदान-प्रदान करते हैं। जब दो परमाणुओं की विद्युत-ऋणात्मकताओं के बीच का अंतर इतना कम होता है कि आयन बनाने के लिए इलेक्ट्रॉन स्थानांतरण नहीं हो पाता है, तो एक सहसंयोजक बंधन बनता है।
जब परमाणु इलेक्ट्रॉनों को साझा करते हैं, तो उनके बीच आकर्षक और प्रतिकारक बलों के एक स्थायी संतुलन को सहसंयोजक बंधन के रूप में जाना जाता है। इन इलेक्ट्रॉन युग्मों को बन्धन युग्म या सहभाजी युग्म के रूप में भी जाना जाता है।
उदाहरण के लिए, ऑक्सीजन (O2), क्लोरीन (Cl2), फास्फोरस ट्राइक्लोराइड (PCl3), इथेनॉल (CH3CH2OH), मीथेन (CH4), ओजोन (O3), हाइड्रोजन (H2), पानी (H2O), हाइड्रोजन क्लोराइड (HCl)
कक्षा 10 के लिए सहसंयोजक बंधन उदाहरण
यहां हमने उनकी संरचनाओं के कुछ उदाहरण दिए हैं।
• क्लोरीन की संरचना (Cl2)
क्लोरीन परमाणु में, Cl2, दो परमाणु एक इलेक्ट्रॉन युग्म साझा करते हैं। सहसंयोजक बंधन इसका कारण बनता है। इसलिए, परमाणु अपने संयोजकता कोश को भरने में सक्षम होते हैं और इसलिए सहसंयोजक बंधों के निर्माण के माध्यम से इलेक्ट्रॉनों को साझा करके एक उत्कृष्ट गैस विन्यास प्राप्त करते हैं।


 JEE Mains Result 2025 Session 2 Live: Sc...
JEE Mains Result 2025 Session 2 Live: Sc...
 NEET City Intimation Slip 2025 Release D...
NEET City Intimation Slip 2025 Release D...
 [Live] CUET UG Date Sheet 2025 @cuet.nta...
[Live] CUET UG Date Sheet 2025 @cuet.nta...










