Copper is one of the most useful element found in the world. The symbol of copper is Cu. Being such an important element, students are asked to study about various aspects of copper including its electron configuration. The electron configuration of copper is necessary to understand in order to understand the properties and behavior of Cu. In this article, we will learn about the Cu Electron Configuration and check the formula for the electronic configuration of copper.
Cu Electron Configuration
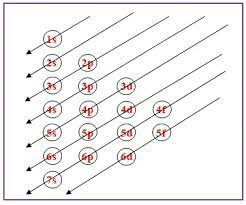
The arrangement of electrons in atoms’ orbitals follows basic principles such as the Pauli exclusion principle and the Aufbau principle, known as electronic configuration. The electronic configuration of copper is a way to represent the distribution of all the electrons present in copper in different orbitals. Copper (Cu), with atomic number 29, has an electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This distinct arrangement is defined by having one electron in the 4s orbital and ten electrons in the 3d orbital, deviating from the usual filling sequence.
Electronic Configuration of Copper
You must have noticed an interesting phenomenon in the electronic configuration of copper. Generally while filling electronics in the orbitals, the orbitals are filled using the energy level rule. By rule, the 4s orbital should have been filled with 2 electrons but you can see that one electron of the 4s orbital goes into 3d orbital to make 10 electrons in it.
The reason for copper’s 3d¹⁰ configuration in the third energy level is due to the enhanced stability of a d subshell that is either half-filled or fully-filled. Copper’s electron arrangement contributes to its high electrical conductivity, making it highly prized in industrial uses like wiring and electronics. The unique arrangement of electrons makes the electronic configuration of copper a frequently discussed subject.
Copper Electron Configuration in Shells
The electronic configuration of copper is typically shown as 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This setup emphasizes how electrons are arranged in various energy levels, known as shells. The first shell is represented by 1s² and 2s², and the second shell is represented by 2p⁶. Meanwhile, the third shell is denoted by 3s² and 3p⁶. The 4s¹ and 3d¹⁰, an unusual assignment, are part of the fourth energy level. The unusual Cu electron configuration in the 4s and 3d orbitals is a result of the energy levels of these orbitals. Students can check the Copper electron configuration in shells in the following picture.
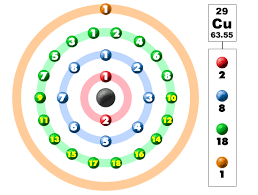
Cu Electron Configuration Formula
Copper has electrons spread out among different shells, with the KLMN shell having the largest amount of electrons. K represents the initial shell, L the subsequent shell, M the third shell, and N the fourth shell in this scenario. The electronic configuration of copper in these shells can be succinctly described as:
- K shell (1s²)
- L shell (2s² 2p⁶)
- M shell (3s² 3p⁶ 4s¹ 3d¹⁰)
- N shell (empty)
The copper electron configuration can also be shown in a different way by taking the noble gas Argon (Ar) as a reference. Check this formula below.
- The atomic number of Copper (Cu) = 29
- Therefore, the expected electronic configuration is [Ar] 3d9 4s²
- However, Copper has an exception in electronic configuration due to the stability concept of orbitals, completely filled and half filled orbitals are the most stable.
- Thus, the observed electronic of copper is [Ar] 3d¹⁰ 4s¹.
Cu Electron Configuration: Properties of Copper
The unique electronic configuration of copper gives this element distinct properties. Copper is a crucial metal recognized for its superb electrical and thermal conduction, establishing it as a crucial component in electrical uses. Its ability to be shaped easily and its ability to resist corrosion have also played a role in its widespread application across various industries, from electronics to construction. Furthermore, its unique reddish-brown hue and shiny metallic appearance also contribute to its popularity in art and decoration.
| Properties Parameter | Details |
| Atomic Symbol | Cu |
| Atomic Number | 29 |
| Atomic Weight | 63.546 amu |
| Classification | Transition Metal |
| Melting Point | 1,984.32°C (3,604.9°F) |
| Boiling Point | 2,562°C (4,644°F) |
| Density | 8.96 grams per cubic centimeter (g/cm³) |
| Color | Reddish-brown |
| Crystal Structure | Face-centered cubic (FCC) |
| Electrical Conductivity | Excellent electrical conductor |
| Thermal Conductivity | High thermal conductivity |
| Magnetic Properties | Non-magnetic (diamagnetic) |
| Malleability | Highly malleable and ductile |
| Luster | Metallic luster |
| Tensile Strength | High tensile strength |
| Corrosion Resistance | Resistant to corrosion, forming a protective oxide layer |
| Chemical Reactivity | Reacts slowly with oxygen and moisture, forming patina |
| Uses | Electrical wiring, plumbing, coins, jewellery, and more |
Copper 29 Electron Configuration
The Copper (Cu) 29 is the normal copper element that has not gained or lost any electrons. Copper has an atomic number of 29, with an electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This arrangement shows how 29 electrons are distributed among the different orbitals in copper’s atomic structure.
Electronic Configuration of Copper 2 Plus (Cu2+) Ion
The Cu2+ ion is formed when the copper element loses two of its electron. When copper gives up two electrons to become a +2 ion, its electronic configuration will be altered. For copper (II) ions (Cu²⁺), the electron arrangement changes to 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸. Losing two electrons means the 3d orbital is now half-filled, leading to a more stable electron configuration.
Electronic Configuration of Copper and Zinc
Copper and zinc are adjacent elements on the periodic table and display varying electronic configurations. The electronic configuration of Copper is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰, whereas zinc, having an atomic number of 30, has the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰. Zinc is a transition metal, unlike copper, because it has a full 4s orbital and does not have the same electron distribution.
The electron configuration of copper is fascinating because of its unique distribution of electrons in the 4s and 3d orbitals. Comprehending the electronic configurations of copper is crucial for understanding its chemical properties and reactivity in different chemical reactions.
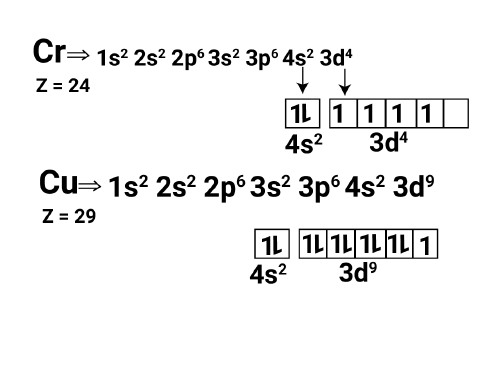
Electronic Configuration of Copper and Chromium
Copper and chromium both display distinct electronic configurations within their individual elements. Chromium has an atomic number of 24 and an electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d⁵. In the same way, copper, which has an atomic number of 29, is configured as 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. These components show deviations from the typical electron filling order because of the enhanced stability associated with half-filled or completely filled d orbitals.
| Related Posts | |
| Electron Configuration | Aufbau Principle |
| Periodic Table | Chemistry Investigatory Project |
| Hybridization Rules | Net Ionic Equations |










 Chemistry Investigatory Project Class 12...
Chemistry Investigatory Project Class 12...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...
 CUET Chemistry Syllabus 2026, Download O...
CUET Chemistry Syllabus 2026, Download O...














