The CUET Chemistry question paper assists students in determining the expected difficulty level of the upcoming exam. Candidates can download the CUET Chemistry question paper 2025 with detailed answers from this page. The CUET UG Chemistry exam took place from May 13 to June 03, 2025. The level of the chemistry paper was moderate in all exam shifts to date and no out-of-syllabus questions have been reported. The CUET UG Chemistry memory-based question paper assists current and upcoming students in knowing the question types, and the difficulty that makes them exam-ready from upcoming shifts.
CUET Chemistry Question Paper
New candidates need to start working on the CUET Chemistry Question Papers from previous years to evaluate their preparation level. A great way to become familiar with the exam structure and recognize question patterns is to work on the CUET Chemistry past question papers. In the meantime, students gearing up for the CUET UG 2025 chemistry exam next year can check the previous CUET Chemistry PYQs from the past three years on this page.
CUET Chemistry Question Paper 2025
The National Testing Agency releases the CUET UG question papers after the exam every year. The CUET Chemistry question paper 2025 memory-based questions released after the exam concludes. The CUET UG 2025 chemistry memory-based questions, along with their answers, are provided on the exam day itself. The NTA releases the CUET chemistry question paper along with the answer key on the official website
CUET UG 2025 Chemistry Question Paper PDF Link
Analyzing the CUET Chemistry Question Paper 2025 is quite useful for candidates whose exams are planned for this year. Through this analysis, students can figure out the CUET Chemistry Question Paper pattern, types of questions, and expected difficulty level, which is crucial for making a strategy. Download the complete paper PDF below –
CUET Chemistry Memory Based Questions 2025
Check CUET UG 2025 chemistry questions based on memory of students below.
| Q.No. | Question | Options |
| Q1 | An alloy of copper, silver, and gold is found to have copper constituting the ccp lattice. If silver atoms occupy the edge centers and gold is present at the body center, the alloy has a formula | A) Cu₄Ag₂Au B) Cu₄Ag₄Au C) Cu₄Ag₃Au D) CuAgAu |
| Q2 | Copper crystallizes in fcc with a unit cell length of 361 pm. What is the radius of the copper atom? | A) 157 pm B) 181 pm C) 127 pm D) 108 pm |
| Q3 | Alkali halides do not show Frenkel defects because | A) Cations and anions have almost equal size B) There is a large difference in the size of cations and anions C) Cations and anions have a low coordination number D) Anions cannot be accommodated in voids |
| Q4 | A solution of urea (mol. mass 56 g mol⁻¹) boils at 100.18°C at atmospheric pressure. If Kf and Kb for water are 1.86 and 0.512 K kg mol⁻¹, respectively, the above solution will freeze at | A) -0.654°C B) 654°C C) -6.54°C D) 54°C |
| Q5 | The solubility of a gas in liquid decreases with an increase in temperature because: | A) Dissolution of a gas in a liquid is an endothermic process B) Dissolution of a gas in a liquid is an exothermic process C) Gases are highly compressible D) All of the above |
| Q7 | Given below are the standard electrode potentials of a few half-cells. The correct order of these metals in increasing reducing power will be: K⁺ | K = –2.93 V, Ag⁺ |
| Q8 | The amount of charge required for the reduction of 1 mol of MnO₄⁻ to Mn²⁺ is | A) 8 × 10⁵ C B) 2 × 10⁶ C C) 8 × 10⁵ C D) 1 × 10⁴ C |
| Q9 | The pressure of H₂ required to make the potential of H₂-electrode zero in pure water at 298 K is | A) 10⁻¹² atm B) 10⁻¹⁰ atm C) 10⁻⁴ atm D) 10⁻¹⁴ atm |
| Q10 | The unit of rate and rate constant are the same for a | A) Zero order reaction B) First-order reaction C) Second-order reaction D) Third-order reaction |
| Q11 | If the volume of the vessel in which the reaction 2NO + O₂ → 2NO₂ is occurring is diminished to 1/3rd of its initial volume, the rate of the reaction will be increased by | A) 3 times B) 6 times C) 27 times D) 30 times |
| Q12 | Assertion: The solubility of any gas in any liquid is an exothermic process. Reason: All gases are highly soluble in any liquid. | A) Both Assertion and Reason are correct, and Reason is the correct explanation of Assertion B) Both Assertion and Reason are correct, but Reason is not the correct explanation of Assertion C) Assertion is correct, and Reason is incorrect D) Both Assertion and Reason are incorrect |
CUET Chemistry Previous Year Question Paper with Solutions
NTA releases CUET Chemistry Question Paper 2025 solutions after the exam conclusion. This helps students calculate their expected scores for the CUET UG 2025 Exam by analyzing their given responses with paper solutions.
CUET Chemist Question Paper 2025 Pattern
Section II of the CUET UG 2025 examination includes 23 domain-specific subjects in which chemistry is the most chosen subject among the PCMB students. According to the CUET UG Notification, the CUET Chemistry Question Paper 2025 will consist of 50 questions, with students having 60 minutes to complete all of them. These previous year’s papers will assist you in understanding the types of questions asked in the CUET Chemistry section. View the detailed CUET Chemistry exam pattern here.
| CUET UG Exam Pattern 2025 Highlights | |
| Particulars | Details |
| Exam Name | CUET UG Chemistry Exam 2025 |
| Mode of the exam |
Computer Based Test (CBT)
|
| Question Type |
Multiple Choice Questions (MCQs)
|
| Total sections |
Section 1 – Language
Section 2 – Domain Subject (Chemistry) Section 3 – General Test |
| Total Questions |
50 questions in each subject
|
| Negative marking | Yes |
| Marking Scheme |
|
CUET Chemistry Question Paper PDF Download
The National Testing Agency conducts the CUET UG examinations in Computer mode, there is no physical paper available. Despite that, Our Adda247 team provides you with the Memory-based CUET Chemistry Question paper with solutions prepared on the basis of student reviews.
The Memory-Based CUET Chemistry Question Papers of the last three years are in downloadable PDF format so that they can download them and start their preparation efficiently.
| CUET Chemistry Question Paper PDF Download |
|
| CUET Chemistry Question Paper 2025 | Available Soon |
| CUET Chemistry Question paper 2024 (15 May Shift 1) | Download PDF (SET 1) |
| CUET Chemistry Question Paper 2023 | |
CUET Chemistry Question Paper with Answers
Practice all the most important Chemistry questions with the answer key for the CUET UG 2025 exam.
1. Arrange the following in decreasing order of number of molecules contained in:
(A) 16 g of O2
(B) 16 g of CO2
(C) 16 g of CO
(D) 16 g of H2
Choose the correct order from the options given below:
Answer: (C), (B), (D), (A)
2. A molecule X associates in a given solvent as per the following equation: X ⇌ (X)n
For a given concentration of X, the van’t Hoff factors was found to be 0.80 and the fraction of molecules was 0.3. The correct value of ‘n’ is:
Answer: 3
3. Which of the following compounds will be repelled when placed in an external magnetic field?
Answer: Na2[CuCl4]
4. The total number of ions produced from the complex [Cr(NH3)6]Cl3 in aqueous solution will be
Answer:4
5. Formaldehyde undergoes Cannizzaro reaction because
(A) It has alpha-hydrogen atom.
(B) It does not have alpha-hydrogen atom.
(C) It does not undergo self-oxidation and reduction on heating with concentrated alkali.
(D) It undergo self-oxidation and reduction on heating with concentrated alkali. Choose the correct answer from the options given below :
Answer: (B) and (D) only
CUET Previous Year Question Paper
6. The gold number range of some of the lyophilic colloids is given below :
A: 0.005 – 0.01, B: 0.15 – 0.25, C: 0.04 – 1.0 and D: 15 – 25.
Which among these can be used as a better protective colloid?
Answer: A
7.The Gatterman-Koch reaction is used in the industrial preparation of benzaldehyde. The electrophile involved in this reaction is
Answer: HCO+
8. The oxidation number of Co in complex [Co(H2NCH2CH2NH2)3]2(SO4)3 is
Answer: 3
7. If 75% of a first onder reaction gets completed) in 32 minutes, time taken for 50% completion of this reaction is
Answer: 16 minutes
8. The correct order of increasing boiling points of the following compounds is: Pentan-1-ol, n-Butane, Pentanal, Ethoxyethane
Answer: n-Butane, Ethoxyethane, Pentanal, Pentan-1-ol
9. The Cu metal crystallises into fee lattice with a unit cell edge length of 361 pm. The radius of Cu atom is:
Answer: 127 pm
10. The correct structure of dipeptide, Gly-Ala (glycyl alanine) is
Answer: H2N – CH2 – CO – NH – CH(CH3) – COOH
11. In the following reaction, identify the product D.
C6H5-OH →(in presence of Zn dust)→ A →(in presence of CH3Cl + anhy. AlCl3)→ B →(in presence of K2Cr2O7 + H2SO4)→ C →(in presence of H2SO4 + HNO3)→ D
Answer: m-Nitrobenzoic acid
12. Arrange the following acids in increasing order of their acidic strengths: HCOOH, FCH2COOH, NO2CH2COOH, CICH2COOH
Answer: HCOOH < CICH2COOH < FCH2COOH < NO2CH2COOH
13. Which of the following compounds will give Hell-Volhard-Zelinsky reaction?
Answer; R – CH2 – COOH
14. Reaction of aniline with conc. HNO3 and conc. H2SO4 at 298 K will produce 47% of
Answer: m-Nitroaniline
15. In the reaction, (CH3)3COCH3 + HI → Products
CH3OH and (CH3)3CI are the products and not CH3I and (CH3)3C – OH. It is because,
(A) in step 2 of the reaction the departure of leaving group (HO – CH3) creates less stable carbocation.
(B) in step 2 of the reaction the departure of leaving group (HO – CH3) creates more stable carbocation.
(C) the reaction follows SN1 mechanism.
(D) the reaction follows SN2 mechanism.
Choose the correct answer from the options given below:
Answer: (B) and (C) only
How to Download CUET Chemistry Question Paper PDF
Adhere to the steps outlined below to learn how to obtain the CUET question paper for Chemistry:
Step 1: Visit the official site of NTA – cuet.nta.ac.in
Step 2: Select the “Downloads” tab.
Step 3: Choose the year, exam category, and document.
Step 4: The display will show links to all the CUET Question Papers.
Step 5: Obtain the question paper, store it, and begin practicing.
CUET Chemistry Question Paper 2024 Analysis
Last year the CUET Chemistry exam was held on 15 May. After taking the exam, students look for solutions to sort down how many questions are correct and how many are wrong, To help students, we provide you with the CUET UG Chemistry Question paper with solutions for all sets hereunder.
CUET Chemistry Question Paper 2024- CUET Chemistry PYQ
Q1. An alloy of copper, silver and gold is found to have copper constituting the ccp lattice. If silver atoms occupy the edge centres and gold is present at the body centre, the alloy has a formula
- Cu4Ag2Au
- Cu4Ag4Au
- Cu4Ag3Au
- CuAgAu
Answer: Option (3)
Q2. Copper crystallizes in fcc with a unit cell length of 361 pm. what is the radius of copper atom?
- 157 pm
- 181 pm
- 127 pm
- 108 pm
Answer:Option (c)
Q3. Alkali halides do not show Frenkel defect because
- Cations and anions have almost equal size
- There is large difference in size of cations and anions
- Cations and anions have low coordination number
- Anions cannot be accommodated in voids
Answer:Option (a)
Q4. A solution of urea (mol. mass 56 g mol-1) boils at 100.18°C at atmospheric pressure. If Kf and Kb for water are 1.86 and 0.512 K kg mol-1, respectively, the above solution will freeze at
(a) -0.654°C
(b) 0.654°C
(c) -6.54°C
(d) 6.54°C
Answer:Option (a)
Q5. The solubility of a gas in liquid decreases with an increase in temperature because:
- Dissolution of a gas in liquid is an endothermic process
- Dissolution of a gas in liquid is an exothermic process
- Gases are highly compressible
- All of the above
Answer:Option (b)
Q6. Assertion: The solubility of any gas in any liquid is an exothermic process.
Reason: All the gas are highly soluble in any liquid.
- Both Assertion and reason are correct and reason is the correct explanation of assertion
- Both assertion and reason are correct but reason is not the correct explanation of assertion
- Assertion is correct and reason is incorrect
- Both assertion and reason are incorrect
Answer:Option (c)
Q7. Given below are the standard electrode potentials of few half-cells. The correct order of these metals in increasing reducing power will be
K +|K = –2.93 V, Ag+|Ag = 0.80 V,
Mg 2+|Mg = –2.37 V, Cr3+|Cr = –0.74 V.
(a) K < Mg < Cr < Ag
(b) Ag < Cr < Mg < K
(c) Mg < K < Cr < Ag
(d) Cr < Ag < Mg < K
Answer:Option (b)
Q8. The amount of charge required for the reduction of 1 mol of MnO4– to Mn2+ is
- 4.8 x 105 C
- 3.2 x 106 C
- 1.8 x 105 C
- 4.1 x 104 C
Answer:Option (a)
Q9. The pressure of H2 required to make potential of H2-electrode zero in pure water at 298 K is
- 10-12 atm
- 10-10 atm
- 10-4 atm
- 10-14 atm
Answer:Option (d)
Q10. The unit of rate and rate constant are same for a
(a) zero order reaction
(b) first order reaction
(c) second order reaction
(d) third order reaction
Answer:Option (a)
Q11. If the volume of vessel in which the reaction 2NO + O2 ? 2NO2 is occurring is diminished to 1/3rd of its initial volume. The rate of the reaction will be increased by
- 3 times
- 6 times
- 27 times
- 30 times
Answer:Option (c)
Q12. KMnO4 acts as an oxidizing agent in alkaline medium. When alkaline KMnO4 is treated with KI, iodide ion is oxidized to ____________.
(a) I2
(b) IO–
(c) IO3–
(d) IO4–
Answer:Option (c)
Q13. The magnetic moment is associated with its spin angular momentum and orbital angular momentum. Spin only magnetic moment value of Cr3+ ion is ___________.
(a) 2.87 B.M.
(b) 3.87 B.M.
(c) 3.47 B.M.
(d) 3.57 B.M
Answer:Option (b)
Q14. Arrange the following in increasing value of magnetic moments.
[Fe(CN)6]4–
[Fe(CN)6]3–
[Cr(NH3)6]3+
[Ni(H2O)4]2+
(a) (i) < (ii) < (iii) < (iv)
(b) (i) < (ii) < (iv) < (iii)
(c) (ii) < (iii) < (i) < (iv)
(d) (iii) < (i) < (ii) < (iv)
Answer:Option (b)
Q15. Zone refining is based on the principle that ___________.
(a) impurities of low boiling metals can be separated by distillation.
(b) impurities are more soluble in molten metal than in solid metal.
(c) different components of a mixture are differently adsorbed on an adosrbent.
(d) vapours of volatile compound can be decomposed in pure metal
Answer:Option (b)
Q16. Which of the following statement is correct?
- Cast iron is obtained by remelting pig iron with scrap and coke using hot air blast
- In the extraction of silver, silver is extracted as a cationic complex
- Nickel is purified by zone refining
- Zr, Ti are purified by the van Arkel method
Answer:Option (d)
Q17. The use of aspartame is limited to cold foods and drinks because
- It is unstable to heat and decomposes at cooking temperature
- It is 500 times sweeter than cane sugar
- It becomes bitter at cooking temperature
- It reacts with the food at cooking temperature
Answer:Option (a)
Q18. Match the column I with column II and mark the appropriate choice.
| Column I | Column II |
|
|
|
|
|
|
|
|
- (A) ? (iii), (B) ? (ii), (C) ? (iv), (D) ? (i)
- (A) ? (ii), (B) ? (iv), (C) ? (i), (D) ? (iii)
- (A) ? (i), (B) ? (iii), (C) ? (iv), (D) ? (ii)
- (A) ? (iv), (B) ? (i), (C) ? (ii), (D) ? (iii)
Answer:Option (d)
Q19. Match the following columns.
| Column I | Column II |
|
|
|
|
|
|
|
|
- (A) ? (i), (B) ? (ii), (C) ? (iii), (D) ? (iv)
- (A) ? (ii), (B) ? (iii), (C) ? (iv), (D) ? (i)
- (A) ? (iii), (B) ? (i), (C) ? (ii), (D) ? (iv)
- (A) ? (iv), (B) ? (i), (C) ? (ii), (D) ? (iii)
Answer:Option (c)
Q20. Which one of the following is not a condensation polymer?
- Nylon-6, 6
- Nylon-6
- Dacron
- Buna-S
Answer:Option (d)
Q21. Synthetic polymer which resembles natural rubber is
- Neoprene
- Chloroprene
- Glyptal
- Nylon
Answer:Option (a)
Q22. Which of the following sets contain only addition homopolymers?
- Polythene, natural rubber, cellulose
- Nylon, polyester, melamine resin
- Teflon, bakelite orlon
- Neoprene, PVC, polythene
Answer:Option (d)
Q23. The secondary structure of protein is represented by
- Alpha-helix structure
- Beta-pleated structure
- Both (a) and (b)
- None of the above
Answer:Option (c)
Q24. Uridine present in RNA is
(a) nucleotides
(b) pyrimidine
(c) purine
(d) nucleoside
Answer:Option (d)
Q25. How many molecules of phenyl hydrazine react with glucose to produce glucosazone?
(a) 3
(b) 4
(c) 2
(d) 1
Answer:Option (a)
Q26. Which of the following is the correct formulation of the entity according to IUPAC?
- (Co(CN)6)3-
- [Co(CN)6]3-
- [Co (CN)6]3-
- [Co(CN)6]-3
Answer:Option (b)
Q27. Identify the correct naming for K3[Fe(CN)6].
- Tripotassium hexacyanidoferrate(III)
- Potassium hexacyanoferrate(III)
- Tripotassium hexacyanoferrate(III)
- Potassium hexacyanidoferrate(III)
Answer:Option (d)
Q28. Pick out the correct statement with respect to [Mn(CN)63-
- It is sp²d² hybridised, tetrahedral
- It is d²sp3 hybridised, octahedral
- It is dsp² hybridised, square planar
- It is sp3d² hybridised octahedral
Answer:Option (b)
Q29. Secondary amines could be prepared by
- Reduction of nitriles
- Hofmann bromamide reduction
- Reduction of amides
- Reduction of isonitriles
Answer:Option (d)
Q30. Identify the correct IUPAC name for the given compound.
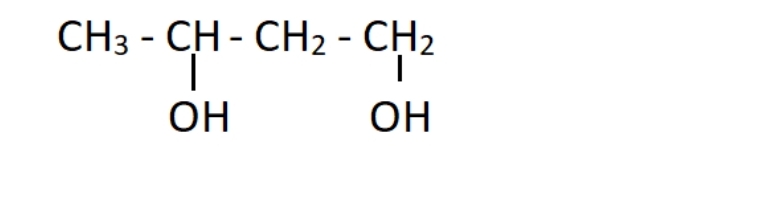 (a) Butane-1,4-diol
(a) Butane-1,4-diol
(b) Butane-diol
(c) Butylene glycol
(d) Butane-1,3-diol
Answer:Option (d)
Q31. Hydrocarbons are formed when aldehydes and ketones are reacted with KOH and ethylene glycol. The reaction is called
(a) Cannizzaro reaction
(b) Clemmensen reduction
(c) Rosenmund reduction
(d) Wolff-Kishner reduction.
Answer:Option (d)
Q32. Which of the following will not give aldol condensation?
(a) Phenyl acetaldehyde
(b) 2-Methylpentanal
(c) Benzaldehyde
(d) 1-Phenylpropanone
Answer:Option (c)
Q33. The correct order of increasing acidic strength is _____________.
(a) Phenol < Ethanol < Chloroacetic acid < Acetic acid
(b) Ethanol < Phenol < Chloroacetic acid < Acetic acid
(c) Ethanol < Phenol < Acetic acid < Chloroacetic acid
(d) Chloroacetic acid < Acetic acid < Phenol < Ethanol
Answer: Option (c)
Q34. Aldehydes other than formaldehyde react with Grignard’s reagent to give addition products which on hydrolysis give
- a) tertiary alcohols
- b) secondary alcohols
- c) primary alcohols
- d) carboxylic acids.
Answer: Option (b)
Q35. Which one is most reactive towards SN1 reaction?
- C6H5CH(C6H5)Br
- C6H5CH(CH3)Br
- C6H5C(CH3)(C6H5)Br
- C6H5CH2Br
Answer:Option (c)
Q36. Which of the following is an example of nuleophilic substitution reaction?
- RX + KOH ? ROH + KX
- 2RX + 2Na ? R-R + 2NaX
- RX + H2 ? R-H + HX
- RX + Mg ? RMgX
Answer:Option (a)
Q37. The compound that responds to Tollen’s test is
- CH3COCH3
- CH3CHO
- CH3COOH
- C2H5OC2H5
Answer:Option (b)
Q38. When treated with alkali and iodine, what substance will not give iodoform test?
- Acetone
- Ethanol
- Diethyl ketone
- Isopropyl alcohol
Answer:Option (c)
Q39. Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is
- Electrophilic elimination reaction
- Electrophilic substitution reaction
- Free radical addition reaction
- Nucleophilic substitution reaction
Answer:Option (b)
Q40. What is the test to differentiate between pentan-2-one and pentan-3-one?
- Iodoform test
- Benedict’s test
- Fehling’s test
- Aldol condensation
Answer:Option (a)
Read the passage given below and answer the following questions: (41-45)
Ozone is an unstable, dark blue diamagnetic gas. It absorbs the UV radiation strongly, thus protecting the people on earth from the harmful UV-radiation from the sun. The use of chlorofluorocarbon (CFC) in aerosol and refrigerator and their subsequent escape into the atmosphere, is blamed for making holes in the ozone layer over the Antarctica. Ozone acts as a strong oxidising agent in acidic and alkaline medium. For this property, ozone is used as a germicide and disinfectant for sterilizing water. It is also used in laboratory for the ozonolysis of organic compounds and in industry for the manufacture of potassium permanganate, artificial silk, etc.
The following questions are multiple-choice questions. Choose the most appropriate answer:
Q41. Which of the following statements is not correct for ozone?
(a) It ixidises lead sulphide
(b) It oxidises potassium iodide
(c) It oxidises mercury
(d) It cannot act as bleaching agent in dry state.
Answer:Option (a)
Q42. Ozone gives carbonyl compounds with
(a) alkyl chloride
(b) alkanes
(c) alkenes followed by decomposition with Zn/ H2O
(d) alcohols followed by decomposition with Zn/H2O.
Answer:Option (c)
Q43. Ozone reacts with moist iodine gives
(a) HI
(b) HIO3
(c) I2O5
(d) I2O4
Answer:Option (b)
Q44. Ozone acts as an oxidising agent due to
(a) liberation of nascent oxygen
(b) liberation of oxygen gas
(c) both (a) and (b)
(d) none of these
Answer:Option (a)
Q45. The colour of ozone molecule is
(a) white
(b) blue
(c) pale green
(d) pale yellow
Answer:Option (b)
Read the passage given below and answer the following questions: (46-50)
Adsorption depends on the nature of the adsorbent. The rough solid surface has more number of pores and adsorb more number of gases than the smooth surface. Most common adsorbents are silica gel, activated charcoal. The extent of adsorption also depends on the surface area of solid. Specific surface area of an adsorbent is the surface area available for adsorption per gram of the adsorbent. The greater the surface area of the solid, the greater would be the adsoprtion. Charcoal is a more effective adsorbent than solid wood. Desorption is a process of removing an adsorbed substance from a surface on which it is absorbed.
Physisorption is non-specific and any gas can be adsorbed. But the gases which are easily liquefiable (e.g., NH3, HCl, CO2) are adsorbed at a faster rate and to a large extent than the gases which are difficult to liquefy (e.g., H2, O2, N2). It depends on the critical temperature. Higher the critical temperature of a gas, more easily liquefiable it is and the more is the rate of adsorption. Chemisorption is specific in nature. Therefore, only those gases can be adsorbed which are capable of forming chemical bonds with the adsorbent.
The following questions are multiple-choice questions. Choose the most appropriate answer:
Q46. Select the correct statement regarding desoprtion.
(a) It is done by cooling or by increasing the pressure applied
(b) It is done by cooling or by increasing the pressure applied.
(c) It is done by heating or by reducing the pressure applied.
(d) It is done by heating or by increasing the pressure applied.
Answer:Option (c)
Q47. Which of the following statements regarding the physical adsorption of a gas on surface of solid is not correct?
(a) On increasing temperature, adsorption increases continuously
(b) Enthalpy changes are negative
(c) It is non-specific in nature
(d) It is reversible in nature.
Answer:Option (a)
Q48. At the same temperature and pressure, select the correct order of adsorption of the following gases on the same mass of charcoal.
(a) SO2 > CH4 > H2
(b) CH4 > SO2 > H2
(c) H2 > CH4 > SO2
(d) CH4 > H2 > SO2
Answer:Option (a)
Q49. Select the correct option among the following when adsorption of a gas on solid metal surface is spontaneous and exothermic.
(a) ΔS increases
(b) ΔS decreases
(c) ΔG increases
(d) ΔH decreases
Answer:Option (b)
Q50. Select the incorrect statement among the following.
(a) Physical adsorption occurs at a low temperature and chemisorption occurs at all temperature.
(b) In physisorption heat of adsorption is low while in chemisorption it is high.
(c) Chemisorption is irreversible and physisorption is reversible.
(d) Magnitude of chemisorption decreases with rise in temperature while physisorption increases with rise in temperature.
Answer: Option (d)











 CBSE Date Sheet 2026 for Class 10 & ...
CBSE Date Sheet 2026 for Class 10 & ...
 CBSE Class 10 Date Sheet 2026, Check 10t...
CBSE Class 10 Date Sheet 2026, Check 10t...
 CUET History Syllabus 2026 (Updated), Do...
CUET History Syllabus 2026 (Updated), Do...







