Table of Contents
To know the difference between orbit and orbital is very necessary for students who study in Class 11 . This blog post is your go-to guide for understanding these concepts in an easy-to-grasp manner. By the end, you’ll be equipped with the knowledge to ace your Class 11 chemistry studies. In this blog post, we’re going to tackle what makes an orbit distinct from an orbital or in simple words what are the basis difference between orbit and orbitals.
Difference Between Orbit and Orbital
The Basis difference between Orbit and Orbital are given below through their definitions. Let’s Check the Difference Between Orbit and Orbital.
Orbit
An ‘orbit’ is the good ol’ path that an electron takes around the nucleus, just like Earth going around the Sun. Imagine a planet orbiting a star—easy enough, right? This was the concept introduced in the early days of atomic theory, and it’s kind of like a planetary model.
Orbital
An ‘orbital’ sounds similar but trust me, it’s a whole different ball game. It’s not a path but a space where there’s a high probability of finding an electron. Think of it as the electron’s hangout zone. Unlike orbits, orbitals come in various shapes and sizes—s, p, d, and f.
Difference Between Orbit and Orbital in Points
Before we dive into the differences, let’s clarify what orbit and orbital mean.
Orbit:
- An orbit is a well-defined, fixed path that an electron follows as it revolves around the nucleus of an atom.
- Orbits are often likened to the rings around Saturn, with distinct levels that electrons occupy.
- Electrons in an orbit are not randomly scattered but maintain a particular energy level.
Orbital:
- An orbital is a three-dimensional region in which there is a high probability of finding an electron.
- Unlike orbits, orbitals don’t have strict boundaries; they describe the electron’s likelihood of being in a specific space.
- Orbitals are classified into different types based on their shape, such as s, p, d, and f orbitals.
Orbit and Orbital Difference in a Nutshell
Now, let’s summarize the primary distinctions between orbit and orbital:
- Nature: Orbits are two-dimensional, while orbitals are three-dimensional.
- Behavior: Electrons follow orbits in a fixed, circular or elliptical path, whereas orbitals describe the electron’s probable location within a three-dimensional space.
- Quantum Mechanics: Orbits are a classical concept, while orbitals are a product of quantum mechanics, providing a more accurate description of electron behavior.
- Energy Levels: Orbits have defined energy levels, while orbitals are associated with energy sublevels (s, p, d, f) and are more specific in terms of energy distribution.
Write the Difference Between Orbit and Orbital Class 11
Imagine a circle for an orbit. Now, for an orbital, think of a cloud around the nucleus. The cloud represents where you’re most likely to find an electron. It’s like an electron’s VIP lounge! This distinction becomes super important in Class 11 Chemistry when you start digging deeper into the atomic structure.
Diagram of Difference Between Orbit and Orbital
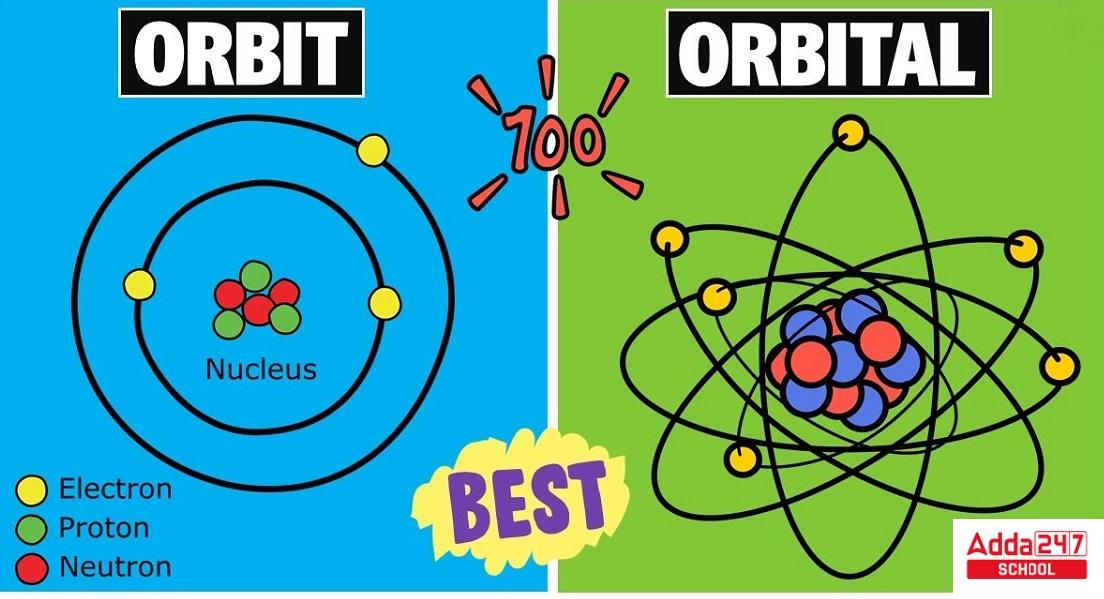
Difference Between Orbit and Orbital in Table
For those who love a good comparison table, this one’s for you:
| Aspect | Orbit | Orbital |
|---|---|---|
| Definition | Path of electron | Space where electron is likely found |
| Shape | Circular | S, P, D, F shapes |
| Energy Levels | Fixed energy | Various energy states |
| Chemical Behavior | Not very explanatory | Useful in predicting chemical behavior |
Difference Between Orbit and Orbital in Chemistry
Now, why the heck would you care about this in Chemistry? Simple: understanding orbitals will make you the Einstein of chemical reactions! Orbitals are vital for explaining why elements react the way they do. They can predict chemical bonding, reactivity, and more. So, the next time you wonder why sodium and chlorine are a match made in heaven, remember—it’s all in the orbitals!
Orbit in Chemistry
Orbits are a concept often associated with the Bohr model of the atom. According to this model, electrons occupy specific orbits with well-defined energy levels. These orbits are numbered and arranged in layers around the nucleus. Electrons can move between these orbits by gaining or losing energy, which is the basis of atomic emission and absorption spectra.
Orbital in Chemistry
Orbitals, on the other hand, are a product of quantum mechanics and provide a more precise description of electron behavior within an atom. They define the probability distribution of finding an electron in a particular region of space. Orbitals are classified into s, p, d, and f orbitals based on their shape and orientation, each with its unique set of quantum numbers.
Difference Between Orbit and Orbitals with Examples
Let’s throw in some real-world examples to cement these concepts in your brain.
- Orbit: Think of Earth going around the sun in a predictable path. Earth doesn’t stray; it keeps to its lane. That’s like an electron in an orbit.
- Orbital: Imagine a buzzing bee around a flower. You can’t pinpoint exactly where the bee will be, but you know it’ll be near that flower. That’s an electron in an orbital!
Difference Between Orbit and Orbitals with Practical
To solidify your understanding, let’s consider a real-world example.
Imagine you’re playing darts, and the dartboard is the nucleus of an atom. The orbits represent the concentric circles on the dartboard, each at a different distance from the bullseye. When you throw the dart, it will land in one of these circles. This scenario illustrates the concept of orbits.
Now, instead of the dartboard, imagine a cloud surrounding the nucleus. This cloud is the orbital, representing the region where the dart is likely to land. There’s no fixed spot; the dart could end up anywhere within this cloud. This analogy mirrors the concept of orbitals, which describe the probability of finding an electron within a three-dimensional space around the nucleus.
ऑर्बिट एवं ऑर्बिटल में अन्तर स्पष्ट करें।
आइए स्पष्ट करें कि कक्षा और कक्षीय का क्या अर्थ है।
की परिक्रमा:
कक्षा एक सुपरिभाषित, निश्चित पथ है जिसका अनुसरण एक इलेक्ट्रॉन परमाणु के नाभिक के चारों ओर घूमते समय करता है।
कक्षाओं की तुलना अक्सर शनि के चारों ओर के छल्लों से की जाती है, जिनमें इलेक्ट्रॉनों का अलग-अलग स्तर होता है।
किसी कक्षा में इलेक्ट्रॉन बेतरतीब ढंग से बिखरे नहीं होते बल्कि एक विशेष ऊर्जा स्तर बनाए रखते हैं।
कक्षीय:
ऑर्बिटल एक त्रि-आयामी क्षेत्र है जिसमें एक इलेक्ट्रॉन पाए जाने की उच्च संभावना होती है।
कक्षाओं के विपरीत, कक्षाओं की सख्त सीमाएँ नहीं होती हैं; वे एक विशिष्ट स्थान में इलेक्ट्रॉन के होने की संभावना का वर्णन करते हैं।
ऑर्बिटल्स को उनके आकार के आधार पर विभिन्न प्रकारों में वर्गीकृत किया जाता है, जैसे कि एस, पी, डी और एफ ऑर्बिटल्स।



 CUET Chemistry Syllabus 2025, Download O...
CUET Chemistry Syllabus 2025, Download O...
 ICSE Class 10 Chemistry 30 Day Preparati...
ICSE Class 10 Chemistry 30 Day Preparati...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...










