Table of Contents
Difference Between Solute and Solvent: The primary difference between solute and solvent is that a solute is a substance that is mixed with a solvent to form a solution. We encounter solutions in everyday activities, thus it is critical to understand the difference between solute and solvent. A solution is a homogeneous mixture of two or more substances. A solution is made up of two components, which are the solute and the solvent. In everyday life, we employ solutions such as lemon juice, sugar solution, and so on. Non-polar polymers are dissolved in non-polar solvents such as toluene in the polymer industry. In this post, we will look at examples of Solutes and Solvents and the difference between solute and solvent.
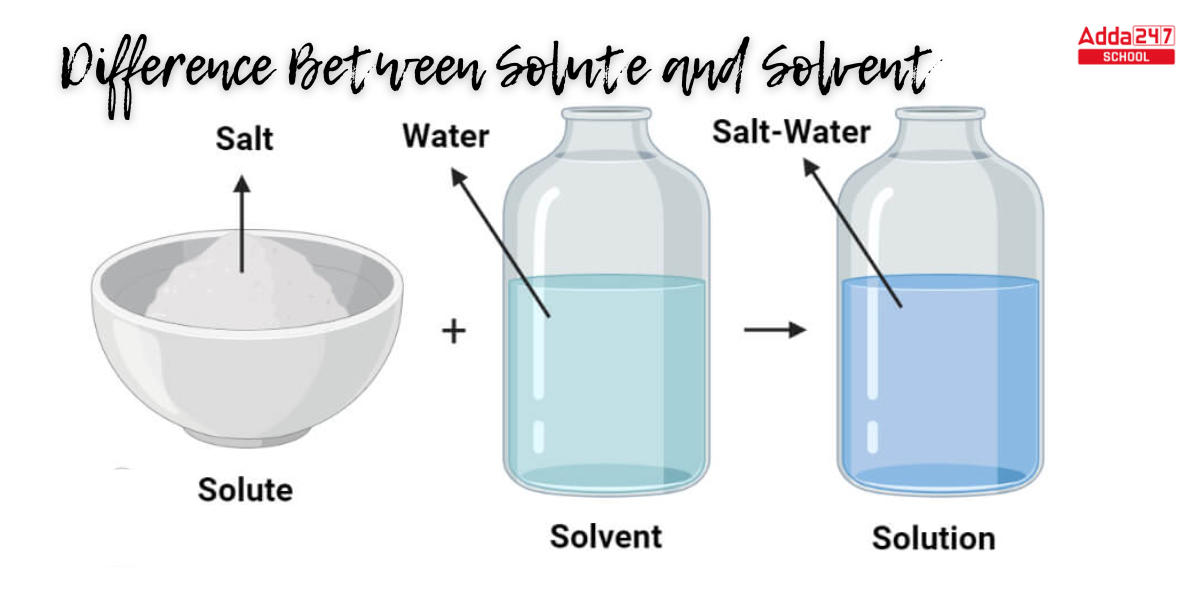
Difference Between Solute and Solvent
What is Solute? Before starting the difference between solute and solvent let’s understand what solute is. A solute is a material that dissolves in water. In a fluid solution, the amount of solvent is larger than the amount of solute. A solute is a component of a solvent that, when dissolved, changes form and loses its original properties. A solute is usually present in lesser quantities in the solvent. A solute can take many different forms. It can exist as a gas, a liquid, or a solid.
Example – In our daily lives, salt, and water are two of the most common instances of solutes. The solute is salt because it dissolves in water.
Types of Solute
Solutes are classed according to their physical and chemical features. Some of these Solutes are addressed more below:
- Ionic Solutes: Ionic Solutes are made up of ions that dissolve in a solvent by dissociating into their constituent ions.
- Molecular Solutes: Molecular Solutes are molecules that dissolve in a solvent due to weak intermolecular interactions.
- Acidic and basic solute: Acidic and basic solutes differ in their ability to give or receive protons (H+ ions).
- Colloidal Solutes: Colloidal solutes are particles that are larger than the molecules of the solvent.
Difference Between Solute and Solvent
What is Solvent? A solvent is characterized as the component that is present in the highest amount in a solution. It is employed in solution to dissolve solutes. Water is regarded as the most prominent Solvent of all other Solvents in Chemistry. Water is also regarded as the universal solvent since it dissolves better than other liquids. Water’s polarity is another factor that contributes to its power. The solvent phase is identical to the solution phase.
Types of Solvent
Solvents are classified into several categories based on chemical characteristics, polarity, and application. The many types of solvents are defined here.
Polar Solvent: Polar Solvents are specific positive charge solvents that have a partial negative charge on one end and a positive charge on the other end, allowing them to dissolve polar solutes.
Non-Polar Solvents: Because they lack partial charges, non-polar solvents cannot dissolve polar solutes.
Aprotic Solvents: Aprotic Solvents lack acidic hydrogen atoms and so cannot give protons.
Protic Solvent: Protic Solvents possess an acidic hydrogen atom that can also give protons.
Difference Between Solute and Solvent in Tabular Form
The solvent, or material that breaks down the solute, isolates the molecules of the solute and distributes them equally. Now we can understand the difference between solute and solvent better from the following table.
| Basis for Comparison | Solute | Solvent |
| Definition | A solute is a material that a solvent may disintegrate into a solution. It can exist as a gas, a liquid, or a solid. | A solvent is the portion of a solution that contains the largest amount of it. Typically, a solvent is a liquid. |
| Quantity | In a solution, the solute has a lower concentration. | A solvent has a greater quantity in a solution. |
| Physical Condition | A solute’s state can be solid, liquid, or gaseous. | The vast majority of solvents are liquids, but some are able to stay gaseous. |
| Current State of the solution | The solution could or could not be in the state of the solute. | Almost, the solution is in the solvent condition. |
| Phase | The solute is the diffused step of a solution. | The solvent is the medium step in the solution that breaks down the solute particles. |
| Boiling point | The solute has a greater boiling point than the solution. | Solvents have a lower boiling point than solutes. |
|
Impact on Solution
|
In charge of modifying the physical characteristics of the solution. | In charge of determining the qualities of the solution. |
| Dependability | The qualities of the solute determine its solubility. | The qualities of the solvent determine solubility. |
| Solubility | A solute’s solubility is determined by factors such as surface area and molecular size. | The qualities of the solvent, such as polarity, determine solubility. |
| Separation | The solute and solvent can be segregated. | The solvent and solution can be separated. |
| Heat transfer | In a solution, heat is transmitted to the solute. | The heat is transferred from the liquid itself to the solution. |
| Examples | Sugar, dissolved carbon dioxide, oxygen, water vapor, and argon are examples of solutes. | Water, Ethanol, Methanol, Acetone, Tetrachloroethylene, Toluene, Methyl acetate, and Ethyl acetate are examples of solvents. |


 NTA NEET Exam Date 2025 OUT, Exam Timing...
NTA NEET Exam Date 2025 OUT, Exam Timing...
 NEET Admit Card 2025 Release Date by NTA
NEET Admit Card 2025 Release Date by NTA
 Anna University Result 2025 OUT at coe1....
Anna University Result 2025 OUT at coe1....










