Electrochemical Series
The electrochemical series is a sequence of half cells grouped in ascending electrode potential order. On the left are oxidants, and on the right are their conjugate reductants. The voltage produced when a half cell is linked to the hydrogen half-cell, indicated in red in the electrochemical series on the right, under typical conditions, is the electrode potential of the half cell. 1.00M solutions, 1 atm pressure, and 25°C are the standard conditions.
Electrochemical Series for Class 10 & 12
| Element | Electrode Reaction | E° (volts) |
| Li | Li+(aq) + e– —> Li (s) | -3.05 |
| K | K+(aq) + e– —> K(s) | -2.925 |
| Ca | Ca2+(aq) + 2e– —> Ca(s) | -2.87 |
| Na | Na+(aq) + e– —> Na(s) | -2.714 |
| Mg | Mg2+(aq) + 2e– —> Mg(s) | -2.37 |
| Al | Al3+(aq) + 3e– —> Al(s) | -1.66 |
| Zn | Zn2+(aq) + 2e– —> Zn(s) | -0.7628 |
| Fe | Fe2+(aq) + 2e– —> Fe(s) | -0.44 |
| Pb | Pb2+(aq) + 2e– —> Pb(s) | -0.13 |
| H | 2H+(aq) + 2e– —> H2(g) | 0 |
| Cu | Cu2+(aq) +2 e– —> Cu(s) | +0.34 |
| Ag | Ag+(aq) + e– —> Ag(s) | +0.80 |
| Au | Au3+(aq) + 3e– —> Au(s) | +1.50 |
Electrochemical Series Trick
To remember the order of elements in the electrochemical series, you can use the mnemonic “Please Stop Calling Me A Zebra, I Like Horses, Can I Call Anybody Silly Gold?” Each capitalized word corresponds to the first letter of an element in the series:
P – Potassium S – Sodium C – Calcium M – Magnesium A – Aluminum Z – Zinc I – Iron L – Lead H – Hydrogen C – Copper A – Silver G – Gold
This mnemonic can help you recall the order of the elements from most reactive (Potassium) to least reactive (Gold) in the electrochemical series.
Electrochemical Series of Metals- Electropositive and Electronegative
Electropositive elements (other than hydrogen) are those that have a higher tendency to lose electrons to their solution. Electronegative elements, on the other hand, are those that gain electrons. They are commonly found after the element hydrogen in the periodic table. In any event, we can figure out the order in which metals will replace one another from their salts by looking at the electrochemical series. As a result, electropositive metals are commonly used to replace hydrogen in acids.
Electrochemical Series for NCERT Class 11 & 12: Applications
Identification of Oxidizing and Reducing agents
The electrochemical series aids in the identification of a good oxidising or reducing agent. All of the substances at the top of the electrochemical series are good oxidising agents, meaning they have a positive standard reduction potential, whereas those at the bottom of the electrochemical series are good reducing agents, meaning they have a negative standard reduction potential. F2, for example, is a strong oxidising agent with a standard reduction potential of +2.87 volts, while Li+ is a strong reducing agent with a standard reduction potential of -3.05 volts.
Calculating EMF of a Electrochemical Cell
The total of the standard reduction potentials of the two half cells is the cell’s standard emf: Half-cells for reduction and oxidation
Eocell = Eored + Eoox
The standard oxidation potential is always represented in terms of reduction potential, as is customary.
- standard oxidation potential (Eoox) = – standard reduction potential (Eored )
- Eocell = standard reduction potential of reduction half-cell – standard reduction potential of oxidation half-cell
Because, The anode undergoes oxidation, while the cathode undergoes reduction. Hence,
- Eocell= Eocathode– Eoanode
Predicting the Feasibility of Redox Reaction
If the free energy change (G) is negative, any redox reaction will occur spontaneously. The following is how the free energy is related to the cell emf:
ΔGo = nFEo
The number of electrons involved is n, the Faraday constant is F, and the cell emf is Eo.
- If Eo is positive, Go can be negative.
- The cell response is spontaneous when Eo is positive, and it acts as a source of electrical energy.
- If the result is negative, the spontaneous reaction will not be possible.
While determining the stability of a metal salt solution when stored in another metal container, the consequent value of Eo for redox reaction is crucial.
Anticipation of the Product
If there are two or more types of positive and negative ions in solution, certain metal ions are discharged or freed at the electrodes in preference to others during electrolysis. In such a competition, the ion with the higher standard reduction potential (stronger oxidising agent) is discharged first at the cathode. When an aqueous solution of NaCl containing Na+, Cl-, H+, and OH- ions is electrolyzed, the H+ ion is preferentially deposited at the cathode (reduction) rather than the Na+ ion, since hydrogen’s reduction potential (0.00 volt) is larger than sodium’s reduction potential (0.00 volt) (-2.71 volt). The anion with the lowest reduction potential will be oxidised at the anode where oxidation takes place. As a result, OH-, which has a standard reduction potential of 0.40 volt, will be oxidised first, followed by Cl-, which has a standard reduction potential of 1.36 volt.
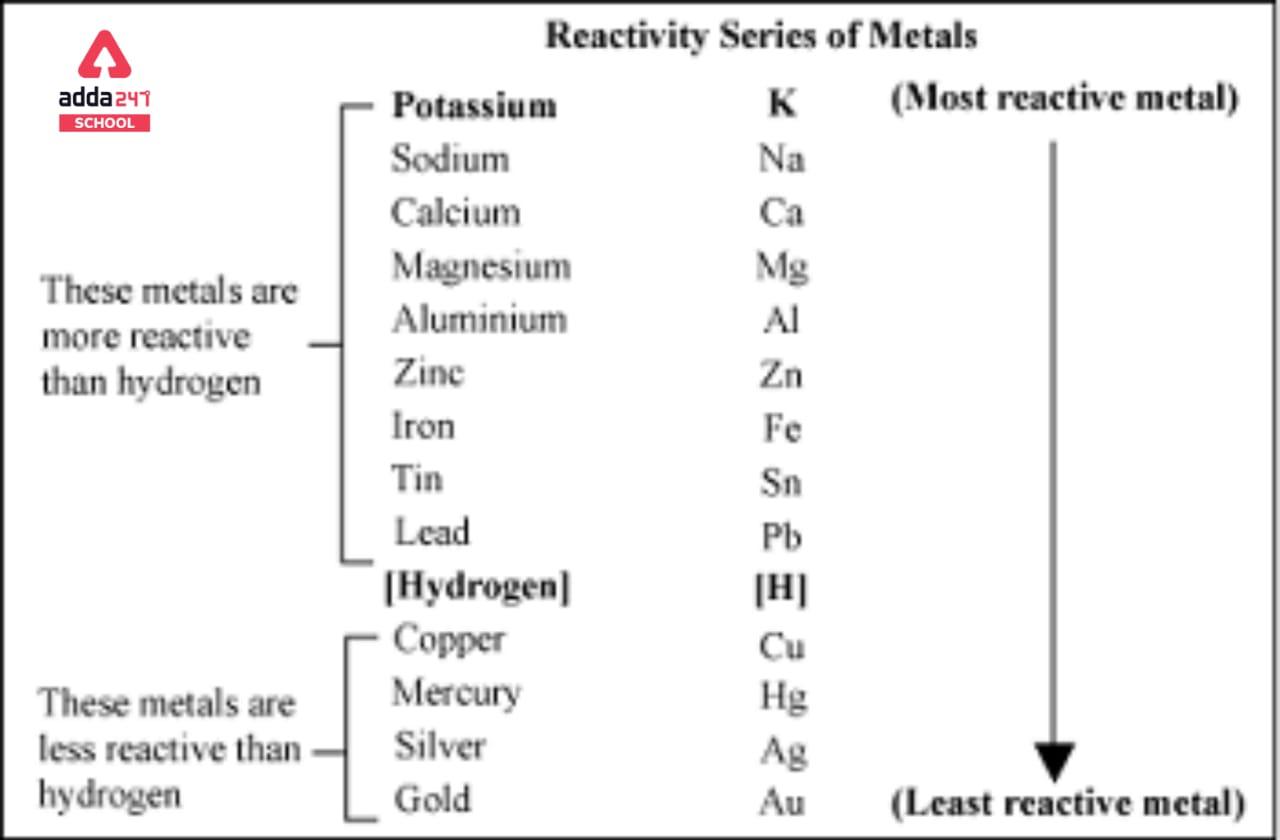
Reactivity Series Trick
Here’s the full reactivity series of metals, starting from the most reactive to the least reactive:
Reactivity Series Trick
- Potassium (K)
- Sodium (Na)
- Calcium (Ca)
- Magnesium (Mg)
- Aluminum (Al)
- Zinc (Zn)
- Iron (Fe)
- Nickel (Ni)
- Tin (Sn)
- Lead (Pb)
- Hydrogen (H)
- Copper (Cu)
- Silver (Ag)
- Gold (Au)
- Platinum (Pt)
In the reactivity series, metals at the top are the most reactive, while those at the bottom are the least reactive. Reactivity refers to how easily a metal reacts with other substances, particularly acids and oxygen. The metals towards the top of the series are highly reactive and tend to react vigorously, whereas metals towards the bottom are less likely to react or do so very slowly. The reactivity series helps us understand the behavior and characteristics of different metals when they come into contact with other substances.
Electronegativity Series
Here’s the electronegativity series of elements, ranging from the highest electronegativity to the lowest:
- Fluorine (F) – Electronegativity: 3.98
- Oxygen (O) – Electronegativity: 3.44
- Chlorine (Cl) – Electronegativity: 3.16
- Nitrogen (N) – Electronegativity: 3.04
- Carbon (C) – Electronegativity: 2.55
- Hydrogen (H) – Electronegativity: 2.20
- Sulfur (S) – Electronegativity: 2.58
- Phosphorus (P) – Electronegativity: 2.19
- Bromine (Br) – Electronegativity: 2.96
- Iodine (I) – Electronegativity: 2.66
EMF Series
The electromotive force (EMF) series, often called the electrochemical series, is a ranking of substances based on their tendency to act as oxidizing or reducing agents in electrochemical reactions. This series helps predict the direction of electron flow in such reactions. More reactive metals, like lithium and potassium, have higher positions as strong reducing agents, while less reactive metals, like gold and platinum, are lower. Similarly, non-metals like fluorine are strong oxidizing agents at the top, while inert gases are at the bottom. This series is fundamental in understanding battery chemistry, corrosion, and other electrochemical processes, aiding in predicting and controlling chemical reactions involving electron transfer.
What is electrochemical series?
In the electronegativity series, elements at the top have the highest electronegativity values, indicating a greater attraction for electrons in a chemical bond. Electronegativity is a measure of an atom’s tendency to attract and bond with electrons in a covalent bond. Fluorine, with the highest electronegativity value of 3.98, is the most electronegative element and has a strong attraction for electrons. As you move down the series, electronegativity values decrease, indicating a lesser ability to attract electrons.
The electronegativity series helps us understand the nature of chemical bonding and the distribution of electrons in compounds. Elements with high electronegativity tend to form more polar bonds, where electrons are shared unevenly, while elements with low electronegativity tend to form more nonpolar bonds, where electrons are shared more equally.
The electrochemical series ranks elements based on their tendency to gain or lose electrons in reactions. More reactive metals, like potassium and sodium, easily donate electrons and stand higher, while less reactive ones, like gold and platinum, are lower. At the top, metals can displace others in reactions, crucial in batteries. At the bottom, metals resist oxidation and corrosion. Similarly, for non-metals, fluorine is highly reactive, while noble gases are inert. This series guides in predicting redox reactions, designing batteries, and understanding corrosion susceptibility, offering insights into the behavior of elements in various chemical processes.


 NEET City Intimation Slip 2025 Available...
NEET City Intimation Slip 2025 Available...
 Maharashtra SSC Toppers List 2025: Who i...
Maharashtra SSC Toppers List 2025: Who i...
 TS Inter Supply Hall Ticket 2025 for IPE...
TS Inter Supply Hall Ticket 2025 for IPE...








