Exothermic Reaction: in the field of chemistry, a chemical reaction is a process that changes one or more compounds (reactants) into one or more unique substances (products). An exothermic reaction is a chemical reaction are releases energy in the form of heat or light. An exothermic reaction releases energy into the environment. Learn more about the exothermic process in this article with examples and detailed explanations.
Exothermic Reaction Class 10
One of the most significant topics in thermodynamics is the exothermic reaction. Exothermic and endothermic reactions are the two categories into which chemical reactions are divided based on the amount of heat involved. Endothermic reactions cause the entire system to warm up because the reactants and the system as a whole absorb heat from the environment. The opposite of endothermic processes, exothermic reactions release energy as heat or light into the environment. One of the best examples of an exothermic reaction, in which energy is released in both the form of heat and light, is lighting a matchstick. Other exothermic reactions include neutralization, fuel-burning reactions, the deposition of dry ice, respiration, etc.
What is Exothermic Reaction?
The term “exothermic” comes from the Greek terms “exo” and “therme,” which indicate “outside” and “heat,” respectively. An exothermic reaction is a reaction or process that releases energy in the form of light or heat. As a result, in an exothermic process, some energy is sent into the environment rather than absorbed from it,
For example, when fluorine gas interacts with barium metal to generate the salt barium fluoride, energy is released and transported to the surroundings. Handwarmers are an exothermic reaction. They emit heat into the environment. Exothermic reactions, on the other hand, do not always produce heat; occasionally the energy is released as light. Glowsticks, for example, emit light without growing in temperature.
Exothermic Reaction Equation
An exothermic reaction occurs when energy is transmitted to the surroundings, and it typically feels warm. These reactions are the inverse of endothermic reactions and can be expressed as follows:
Reactant → Products + Heat Energy/ Light Energy
This reaction has a negative change in enthalpy (ΔH), which is why we refer to it as ΔH<0. When an exothermic reaction occurs, the net energy released is always more than the energy required for initiating it.
Also check: Endothermic Reaction Examples & Equations
What is the Source of the Exothermic Heat Energy?
Have you ever wondered where exothermic heat energy comes from? An exothermic reaction emits energy into the surrounding environment. The energy held in the chemical bonds of the reactant molecules is larger than the energy stored in the chemical bonds of the product molecules, resulting in heat. The situation is inverted in endothermic chemical reactions: more chemical energy is stored in the bonds of the product molecules than in the bonds of the reactant molecules.
Explain Exothermic Reaction with Examples
Any material that is burned undergoes chemical transformation and releases heat energy during the reaction. Exothermic reactions occur when the system (where the reaction is taking place) transfers energy into the surrounding environment in the form of heat. Thus, in an exothermic process, energy is transferred into the environment rather than taken from it, as in an endothermic reaction. The change in enthalpy ( ΔH)) in an exothermic reaction is negative.
The entire amount of energy in a given chemical system can be calculated, but it is very challenging to quantify. As a result, the energy change (or *enthalpy change, represented by H) is instead measured. The following equation describes the relationship between the value of ΔH and the reaction’s bond energies.
ΔH = (energy used in product bond formation) – (energy released when reactant bonds are broken)
As a result, it is clear that an exothermic reaction will always have a negative value for the change in enthalpy, i.e. ΔH < 0.
ΔH = Hproducts – Hreactants < 0 [where a larger value (the greater reactant energy) is subtracted from a smaller value (the lesser product energy). As an illustration, when hydrogen burns,
2H2 (g)+ O2 (g)→ 2H2O (g)
ΔH⚬ = −483.6 kJ/mol
An exothermic reaction’s measured heat energy is converted to “ΔH” in Joule per mole (formerly cal/mol)
*Enthalpy: In a thermodynamic system, energy is measured by enthalpy. Enthalpy is the total heat content of a system, which is equal to the system’s internal energy in addition to the product of volume and pressure.
How to Measure Enthalpy Change
Bomb calorimeters are excellent tools for measuring the enthalpy change of combustion reactions. When a calorimeter is used to measure the heat produced by a chemical process, the net quantity of energy in the form of heat that flows through the calorimeter is equal to the system’s total energy change minus one.
Exothermic Reaction Graph
In chemistry, we can present the energy change in a chemical reaction through a graph. In the exothermic reaction graph, the x-axis depicts reaction time, and the y-axis represents reaction potential energy. As per the definition of the endothermic reaction, we can see from this graph that the energy of the reactants is greater than that of the products. It denotes that H is negative(ΔH<0). which makes the reaction is exothermic.
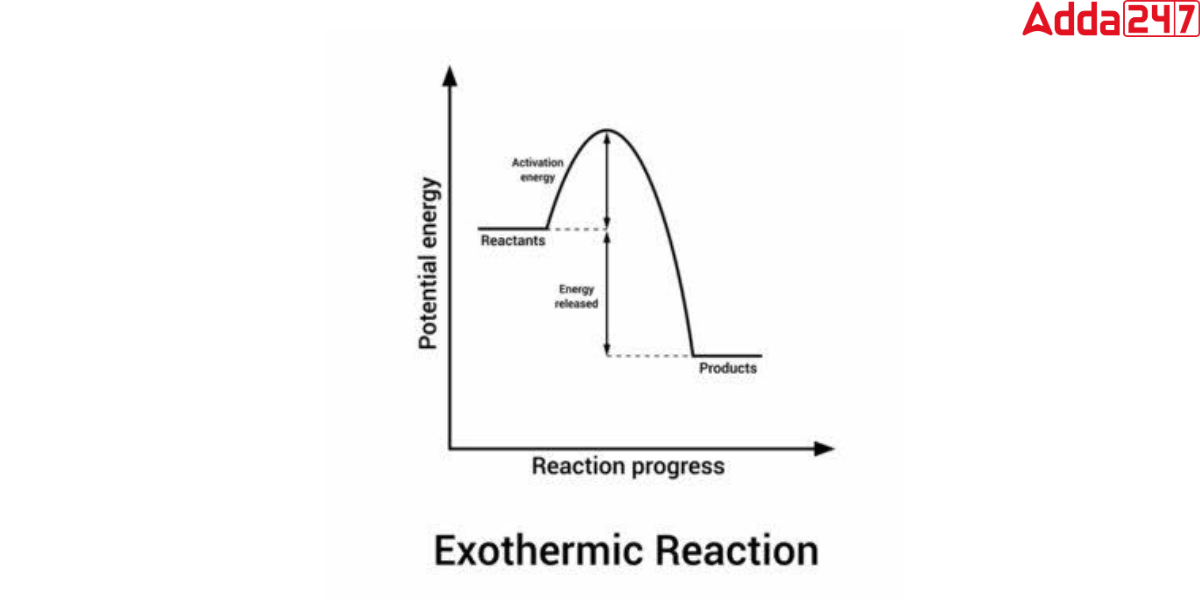
Exothermic Reaction Examples with Equation
Exothermic reactions are seen everywhere, from candle burning to nuclear fusion events. Here are a few notable real-life examples of exothermic reactions:
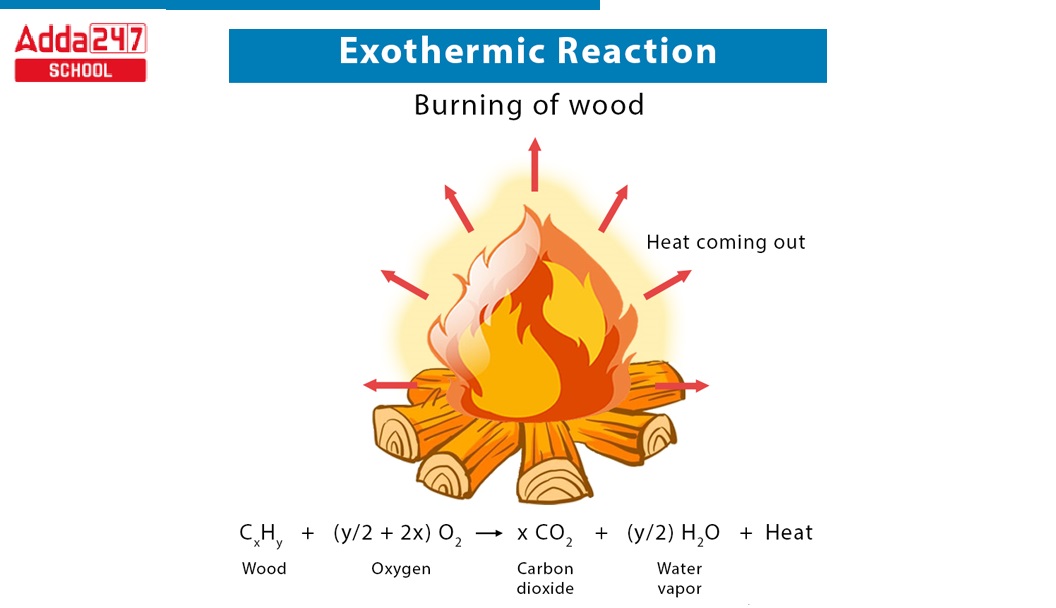
- Burning/Lighting of Candle: An example of an exothermic reaction is the burning of a candle. When you place your finger close to the flame, you can feel the energy released into the atmosphere as the candle burns. The flame is hot, indicating that heat is being discharged into the environment.
- Uranium-235 Nuclear Fission: The nuclear fission of one atom of uranium-235 produces more than 2.5 million times the energy provided by coal burning. The fission of one atom can set off a chain reaction in which a neutron from the fission of one uranium-235 atom strikes another atom, causing nuclear fission.
- Combustion Reaction: Combustion is an exothermic reaction in which fuel is reduced in the presence of an oxidising agent. This oxidising agent is typically oxygen found in the atmosphere. These reactions provide a lot of energy in the form of heat, but they also produce certain byproducts like smoke.
- Methane combustion: Exothermic reactions include the combustion of natural gas methane. The chemical reaction for the exothermic reaction is as follows
CH4 + O2 → CO2 + H2O + Heat
- Nitroglycerin detonation: Nitroglycerin is a combustible chemical with exceptionally volatile physical qualities that rapidly catches fire. Nitroglycerin decomposes during combustion, releasing a significant quantity of energy. Because of this, it is a popular element in explosives and dynamite. When nitroglycerin is detonated, the fuel in the surrounding area burns, resulting in an exothermic reaction.
Is Respiration an Exothermic Reaction?
Yes, Respiration is an exothermic reaction. Respiration is the transfer of oxygen from the environment to the cells within tissues, while carbon dioxide is eliminated from the body, and back to the environment. During the event of respiration, glucose (C6H12O6 ) reacts with oxygen ingested by humans to produce carbon dioxide, water, and energy. The corresponding chemical equation for respiration is as follows:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
When energy is transported to the surroundings, an exothermic reaction occurs, and it often feels warm. This energy generated in the reaction implies that the respiration process is an exothermic reaction.








 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...
 CUET PG Crash Course 2026: Subject-Wise ...
CUET PG Crash Course 2026: Subject-Wise ...














