Table of Contents
Fermentation is a metabolic process that results in chemical changes in organic substrates, and zymology is the study of fermentation. Enzymes are responsible for chemical changes. It is narrowly defined in biochemistry as the extraction of energy from carbohydrates in the absence of oxygen. It could also apply to any method in which the action of microbes causes the desired alteration in a food or beverage.
Fermentation Meaning
Fermentation is the most common way for bacteria to produce adenosine triphosphate. Fermentation has been utilized to make foods and beverages by humans since the Neolithic period, and the process is employed for both preservation and the production of alcoholic beverages. Animals, including humans, undergo fermentation in their gastrointestinal tracts.
What is Fermentation for Class 8 and 10?
Fermentation is the breakdown of a chemical into a simpler substance, and microorganisms such as yeast and bacteria are commonly involved in the process. Microorganisms play a key role in the fermentation process, which results in the production of beer, wine, bread, kimchi, yoghurt, and other foods.
Fermentation is derived from the Latin verb fermentare, which means “to ferment.” Which literally means “to leaven.” To make bread rise, you mix a leavening agent with water to “wake up” dried yeast, which then begins “eating” the sugar in the dough. It then begins to off-gas alcohol. This is also a fermentation process. Even after grape juice is transformed into wine, the fermentation process is complete.
Fermentation Process
Fermentation is a technique for extracting energy from molecules that is used by all bacteria and eukaryotes. As a result, it is thought to be the oldest metabolic route, ideal for prehistoric settings.
Yeast may be found in practically any habitat that can host microorganisms, and it transforms sugar-rich compounds into ethanol and CO2.
Basic fermentation mechanisms are found in all cells of higher species, and fermentation occurs in a Mammalian muscle during periods of heavy exercise. Lactic acid is produced during a high-intensity activity when oxygen supply becomes limited. Invertebrates produce succinate and alanine as a result of fermentation. Fermentative bacteria are necessary for the formation of methane, as well as hydrogen, carbon dioxide, formate, acetate, and carboxylic acids. The carbon dioxide and acetate are then converted to methane by microbe consortiums, acetogenic bacteria oxidise the acids, and methanogens convert acetate to methane.
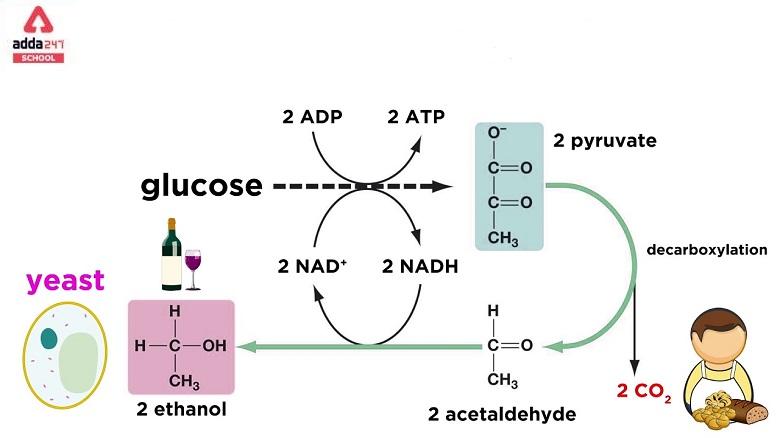
Fermentation Diagram
The fermentation Diagram helps you to understand the full process of fermentation. The fermentation diagram is given below.
Fermentation Products
Fermentation is a natural process that has been used to create items like wine, mead, cheese, and beer even before the biochemical process was fully understood. Louis Pasteur was the first scientist to explore fermentation in the 1850s and 1860s. He demonstrated that live cells induce fermentation. Louis Pasteur, on the other hand, was a failure. He was unable to extract the enzyme that causes fermentation from yeast cells. Eduard Buechner, a German scientist, powdered yeast and extracted fluid from it in 1897. He even discovered that the liquid was capable of fermenting a sugar solution. Buechner’s experiment is regarded as the origin of biochemistry research. He was awarded the Nobel Prize in Chemistry in 1907.
Fermentation Reaction
Fermentation Reaction is a very useful reaction in chemistry for board exams and for use in industry or in practical life.
Fermentation Examples- किण्वन के उदाहरण
Fermentation-Formed Products Examples are mentioned below:
- Sauerkraut, kimchi, and pepperoni are examples of lactic acid-containing foods.
- Yeast is used to leaven bread
- Yogurt
- Cheese
- Beer
- Wine
- Treatment of sewage
- Some alcohol manufacturing industries, such as biofuels
Fermentation of Alcohol
One glucose molecule is transformed into two ethanol molecules and two carbon dioxide molecules during ethanol fermentation, which is used to raise bread dough. The carbon dioxide creates bubbles, which stretch the dough into foam. The intoxicating agent in alcoholic beverages is ethanol. Furthermore, ethanol is produced via the fermentation of feedstocks such as sugarcane, corn, and sugar beets. Ethanol is mixed with gasoline.
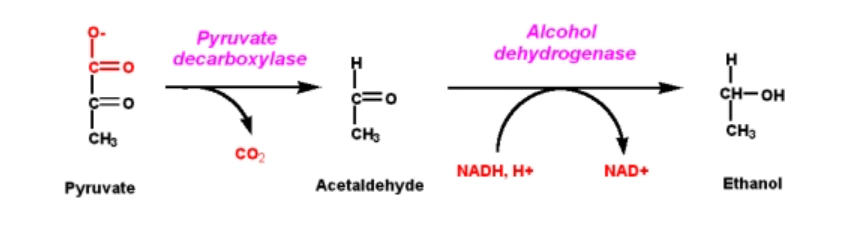
A glucose molecule splits into two pyruvate molecules before fermentation, and the energy from this exothermic event is used to link inorganic phosphates to ADP. This ADP turns NAD+ to NADH by converting it to ATP. The pyruvates are broken down into two acetaldehyde molecules, which emit two carbon dioxide molecules as waste products, and the acetaldehyde is then converted into ethanol utilising the energy and hydrogen provided by NADH. In addition, NADH is oxidised to NAD+. The enzymes pyruvate decarboxylase and alcohol dehydrogenase perform the process.
Example of Fermentation of BEER
Fermentation is a metabolic process that allows a cell to produce energy without the use of oxygen. One of the most well-known examples of fermentation is the process by which yeast converts sugars into alcohol and carbon dioxide during the making of beer and wine.
Beer Fermentation Example
Ingredients:
- Malted barley
- Hops
- Water
- Yeast (Saccharomyces cerevisiae)
Steps:
- Mashing: Malted barley is mixed with hot water to create a “mash.” This process helps to convert the starches in the barley into simpler sugars.
- Boiling: The liquid part of the mash, known as “wort,” is separated and boiled with hops for flavor and bitterness.
- Cooling: The wort is then cooled to a temperature that is suitable for yeast fermentation.
- Fermentation: Yeast is added to the cooled wort in a sterile environment. The yeast consumes the sugars and produces alcohol and carbon dioxide as by-products.
- Aging: The beer is allowed to age in a controlled environment to develop flavors.
- Packaging: After reaching the desired alcohol content and flavor, the beer is filtered and packaged into bottles, cans, or kegs for distribution.
Chemical Reaction:
The simplified chemical equation for the fermentation of glucose by yeast is:
is glucose (sugar)
is ethanol (alcohol)
is carbon dioxide
This example demonstrates how fermentation is used to produce beer, one of the oldest and most commonly fermented beverages.
Related Post:
- Lens Formula: Derivation, Magnification, Power Of Lens
- Diversity Meaning
- Father Of Geography
- Greenhouse Gases
- Vermicompost / Vermicomposting: Meaning, Process, Price
- The Biggest/Largest Country In The World
- Respiratory System: Parts, Function, Organs And Diseases
- Folk Dances Of India
- Hindi Alphabets, Varnamala & Letters (हिंदी वर्णमाला)


 CUET Chemistry Syllabus 2025, Download O...
CUET Chemistry Syllabus 2025, Download O...
 ICSE Class 10 Chemistry 30 Day Preparati...
ICSE Class 10 Chemistry 30 Day Preparati...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...










