Table of Contents
Glucose Formula: The glucose word is derived from the Greek root word “glukus”, referring to “sweet.” Simple glucose has 6 carbon atoms and one aldehyde group. It is a monosaccharide, which is a synonym for simple sugar. The glucose formula is C6H12O6 (Chemical formula). Dextrose is another name for it. Glucose comes in two varieties: open-chain and ring construction. In this article, we will dive into the chemical structure of glucose, glucose formula, glucose formation, glucose properties and applications, and much more.
Definition of Glucose C6H12O6 (Sugar)
Glucose C6H12O6 is a sugar that plants make during photosynthesis and circulate as an energy source in the blood of humans and other animals. It is one of the three monosaccharides used by the organism. It is the only one that produces ATP (Adenosine Triphosphate) directly. It is produced in animals’ livers and kidneys. It is found in plants in fruits and various plant components. D- Glucose is the most common kind of glucose found in nature. Dextrose is a molecule of D-glucose.
What is Glucose?
Jean Baptiste Dumas invented the term glucose in 1838. Andreas Marggraf, a German scientist, extracted glucose from raisins in 1747. It may appear as a liquid or as a solid. Glucose is water soluble as well as acetic acid soluble. It has no scent and tastes delicious. Glucose is also known as aldohexose because it includes six carbon atoms and an aldehyde group. Starch, the primary energy-reserve carbohydrate in plants, is composed of thousands of linear glucose units. Cellulose is another key glucose-based compound.
Glucose Molecular Mass
The molecular formula for glucose is C6H12O6. It is generally available as white power. The exact molecular mass of glucose is 180.156 g/mol.
Glucose Formula (Sugar Formula)
- Glucose has the chemical formula C6H12O6 or H-(C=O)-(CHOH)5-H. Its empirical or simplest glucose formula is CH2O, which implies that the molecule has two hydrogen atoms for every carbon and oxygen atom.
- 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms make up glucose. Because of the tetrahedral geometry of carbons, the -OH on carbon number 5 transforms into the ether bond, which seals the ring with carbon number 1. As a result, a ring with six members (five carbons and one oxygen) is formed.
- Other names for glucose include dextrose, blood sugar, maize sugar, grape sugar, and its systematic IUPAC designation (2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanal.
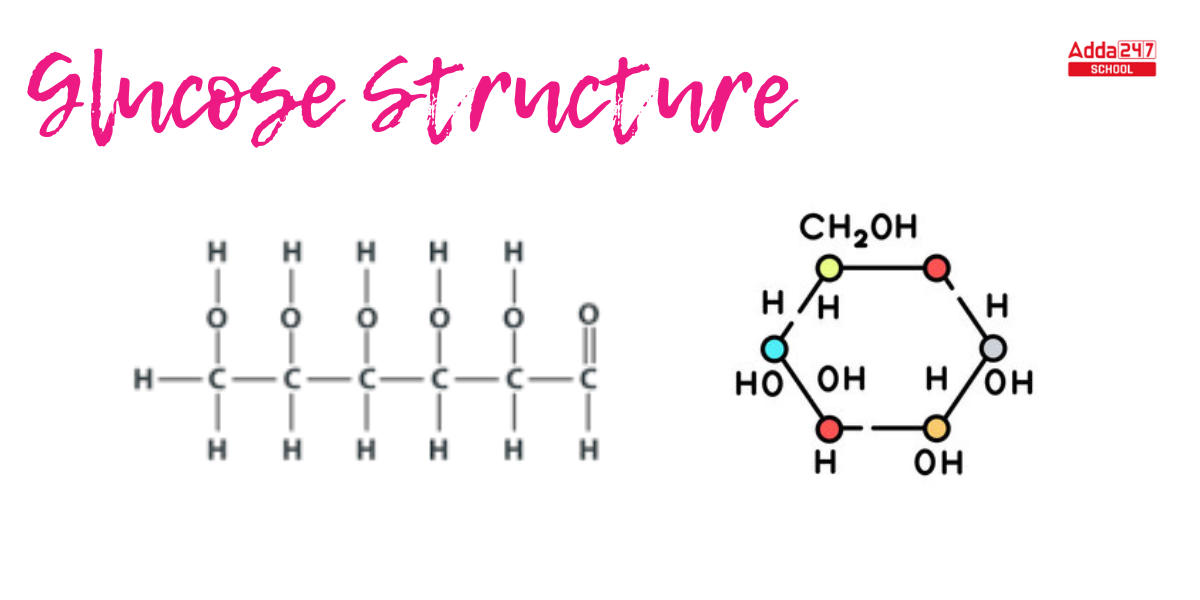
Glucose Structure
Based on the study of elements in glucose and the molecular mass of glucose, the molecular formula of glucose is C6H12O6. In its open-chain form, glucose has a backbone that is not open or branched and is composed of six carbon atoms.
- 6-carbon unbranched chain: Glucose has an unbranched six-carbon chain, as evidenced by the fact that it completely decreases when coupled with concentrated hydrogen iodide and red phosphorus to generate n-hexane.
- 5 OH groups: The presence of 5 OH groups: Because glucose is a stable molecule, no two OH groups can bond to the same carbon. As a result, five OH groups can be found on diverse carbons. When glucose interacts with acetic anhydride, pentadactyl derivatives are produced, signifying five hydroxyl groups. When glucose reacts with hydroxylamine, an oxime is generated, indicating the presence of a carbonyl group.
- Terminal CHO : When the glucose molecule is moderately oxidised with bromine water, it converts into glucose acid, which is subsequently reduced with a large amount of HI to make hexanoic acid.
- Because glucose is a 6-carbon straight chain with a terminal CHO, the five OH groups can be attached to each of the other five carbons to generate an open-chain glucose structure. Supplyingsupplying hydrogen atoms to these carbon atoms, this fits the four valencies of the carbon atom.
How is Glucose Formed?
Glucose is primarily obtained from sucrose (cane sugar) and starch. the process of extracting glucose from sugar can in the labortory technique is discussed below.
Preparation of Sugar from Sucrose or cane sugar (laboratory method)
Sucrose, having the formula C12H22O11, is a disaccharide.
- In the laboratory, glucose is made by hydrolyzing sucrose with dilute hydrochloric acid or dilute sulfuric acid for roughly two hours.
- Sucrose hydrolysis yields one molecule of glucose and one molecule of fructose. Because glucose is almost insoluble in alcohol and hence crystallizes first, alcohol is added during cooling to separate it from the fructose.
- Because fructose is more soluble than glucose, it remains in the solution. Glucose crystals are separated by filtering and refined by recrystallization.
C12H22O11 + H2O —–→ C6H12O6 + C6H12O6
Sucrose (H+,dil HCL) Glucose + Fructose
Preparation of Sugar from Starch
It is a polysaccharide that produces glucose when cooked with dilute H2SO4 at 393 K under 2 to 3 atmosphere pressure.
(C6H12O5)n + n H2O nC6H12O6
Starch Glucose
Glucose Chemical Properties
Physical Properties of Glucose Molecule –
- Glucose is available as a solid or a liquid.
- It is in the delicious taste.
- Glucose dissolves in water.
- It appears to be colourless and transparent.
- Glucose has a boiling point of 294.8°F (146°C).
- At 760 mmHg, the melting point of glucose is 527.1+ or -50°C.
- After acetylation of glucose with acetic acid, which yields glucose pentaacetate, the existence of a -OH group is established.
Glucose’s Chemical Properties
- C6H12O6 is the chemical formula for glucose.
- D-glucose is the IUPAC designation for glucose.
- Glucose has a density of 1.54g/cm3.
- Glucose has a molecular weight of 186.16g/mg.
- When hydrogen cyanide is added to glucose, it interacts with hydroxylamine and cyanohydrins to generate oxime. The fact that there is of the carbonyl group in glucose can be confirmed by this reaction.
- Because of its monosaccharide content, it is known as simple sugar. It is disorganised.
Importance Uses of Glucose
Some important roles of glucose in the human body are discussed below.
- Glucose is also a component of bigger structural molecules in the body, such as glycoproteins and glycolipids.
- It is used to treat hypoglycemia which is low blood sugar.
- Glucose is given to individuals who are really ill and unable to eat because it supplies carbohydrate calories.
- If blood sugar levels drop, brain function may suffer. Headache, dizziness, disorientation, poor focus, anxiety, irritability, restlessness, delayed speaking, and poor coordination are all common hypoglycemic brain symptoms.
- Glucose is used to treat high potassium levels in the blood (hyperkalemia).
- During the Activity, glycogen quickly breaks down to provide glucose. During activity, muscle tissue absorbs a substantial amount of glucose from the bloodstream.
- It is Utilized in the synthesis of compounds as a precursor.



 NEET UG 2025: Is NEET Previous years Que...
NEET UG 2025: Is NEET Previous years Que...
 JEE Mains Session 2 Result 2025 OUT, Sco...
JEE Mains Session 2 Result 2025 OUT, Sco...
 TS Inter Results 2025 Date for TSBIE 1st...
TS Inter Results 2025 Date for TSBIE 1st...










