The Gold formula or chemical symbol is Au, which signifies aurum. Gold is a chemical substance that occurs naturally. In the periodic chart, it has the atomic number 79. It is a bright yellow material that is dense, supple, flexible, and stretchy. It is regarded as a precious metal that is utilized in jewelry, and art, and as an investment choice. Gold is a Chemical Allergen that has been Standardised. Gold has a physiological effect by increasing histamine release and cell-mediated immunity. Let us discover more about gold’s structure, composition, chemical and physical properties, uses, and more.
Gold Formula
A molecular formula is an expression of an element’s atoms in a compound. Every component in the periodic table is represented with a symbol that allows for easy identification. The symbol that symbolizes these elements in the chemical formula is the first or first two letters of their names. The Gold formula is Au and the atomic weight is 197, it’s a yellow metallic element.
Gold is a malleable and ductile metal that can be formed into wires and sheets. Gold is not a very reactive metal. It has two distinct oxidation states: the Auric oxidation state and the Aurum oxidation state. It is malleable and ductile, which makes it unique. That is, its malleability enables it to be formed into thin sheets, while its ductility enables it to be pulled into fine wires.
Chemical Formula of Gold
The chemical formula of gold is “Au,” which is derived from the Latin word for gold, “aurum.” Gold is represented by the element symbol Au in the periodic table, and its atomic number is 79. Gold is a metallic element known for its beautiful, lustrous appearance and is often used in jewelry and various industrial applications.
Gold Formula Sturcture
Gold has a crystalline structure. The crystal structure of gold is face-centered cubic (fcc). This configuration produces a highly symmetric and densely packed structure.
- Atoms are organized in a cube (fcc) in a face-centered cubic structure, with an atom at every edge of the cube and one in the center of each face of the cube.
- In the case of gold, a gold atom occupies each corner of the cube. Each face of the cube has one gold atom in the center. The fcc crystal structure also adds to the excellent reflectivity and luster of gold.
This arrangement is responsible for gold’s distinctive metallic qualities, such as flexibility and rigidity, which enable it to be easily bent and reshaped without cracking. The Gold formula structure is shown below.
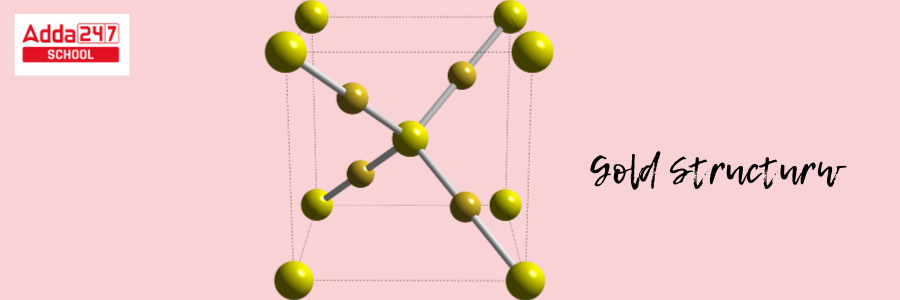
Atomic Structure of Gold
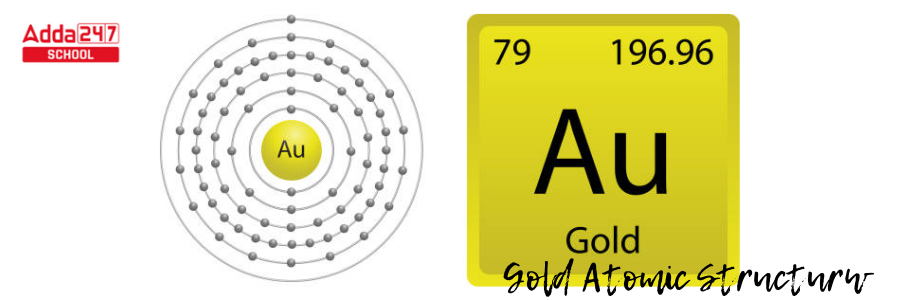
- The mass of gold is 197 atomic mass units. Subtract the atomic mass from the number of protons. 197 – 79 = 118 in gold. Gold contains 118 neutrons.
- The nucleus of gold, then, has 79 protons and 118 neutrons.
- The extra neutrons reduce the attraction between positively charged protons. The nucleus is held together by nuclear forces.
- There are six degrees/ level of energy in a gold atom. The 79 electrons will occupy the orbitals in those levels of energy to the extent that each orbital can contain them.
- The number of electrons that fit in each energy level from the first to the sixth can be estimated using 2n2, where n is the energy level.
- The initial energy level, n = 1, using 2n2, is 2(1)2; meaning, it can hold two electrons. The first six energy levels can each carry 2, 8, 18, 32, 50, and 72 electrons.
- Gold, as an electron-filling anomaly, will fill levels from the lowest to the highest energy level, and the electron numbers are 2, 8, 18, 32, 18, and 1.
Gold Formula – Natural Occurrence
There is hardly a trace of gold in the Earth’s rocky layer, about 0.005 parts per million.
- Gold can be found as chunks or flakes in a variety of geological formations, including rocks, veins, and alluvial sediments.
- It can also be found in natural blends with silver, producing alloys with other metals such as copper and palladium, and as mineral inclusions such as pyrite.
- Gold is frequently discovered in conjunction with copper and lead deposits. Large quantities of gold-rich rock, large enough to be termed ores, are uncommon.
- Gold deposits are classified into two types: hydrothermal veins, where it is frequently found accompanying quartz and pyrite, and placer deposits, both stabilized and unconsolidated, which form as a result of weathering of rocks.
Preparation of Gold
Gold nanoparticles should be manufactured on a large scale and easily functionalized by chemical compounds or particular ligands or biomolecules. It is not practical to produce gold in a laboratory for commercial purposes due to the huge cost.
- The influence of varying doses of reducing agent (NaBH4) on the size of particles, variation in size, and shape was evaluated in gold nanoparticles synthesized (for research).
- Gold can be created in a laboratory using nuclear processes, but it is prohibitively expensive and impractical.
- One method of producing gold is to bombard mercury with neutrons, which produces radioactive gold isotopes that decay into stable gold.
- Another method is to transmute bismuth with neon or carbon ions, which yields radioactive gold isotopes that decay into stable gold. However, these processes necessitate a significant quantity of energy, equipment, and safety precautions, and the total quantity of gold produced is insignificant in comparison to the cost. As a result, commercially producing gold in a lab is not viable.
Properties of Au
The face-centered cubic structure of gold is an important factor in its unique combination of physical and chemical properties, which makes it highly desired for a wide range of uses ranging from jewelry to electronics and beyond. Some of the properties of gold are mentioned below.
- Gold has an atomic Atomic Number of 79.
- While copper and silver are well-known for their outstanding heat and electrical conductivity, gold connectors shine due to their tarnishing resistance.
- Gold belongs in the following groups, periods, and blocks: Group 11, 6th period, d-block.
- Gold’s electronic configuration is [Xe]. 4f14 5d10 6s1
- Gold can be stretched into an 80-kilometer-long wire with a thickness of five microns (or five-millionths of a meter) and a diameter of 0.20 millimetres.
- Gold has a density of 19.30 grams per cubic centimeter (g/cm3).
- Gold has a boiling point of 3243 Kelvin (K).
- Gold has a melting point of 1337.33 K.
- Because gold is extremely reflective of both heat and light, it is an excellent material for coating the visors of astronauts’ space helmets. This thin, partially transparent covering of gold lowers solar glare and heat while letting astronauts see over it.
Gold Formula and Uses
Gold nanoparticles have been employed in a wide range of applications, including electronics, biosensors, in vivo biomedical imaging, and in vitro biomedical diagnosis.
- Gold was first used to make jewelry roughly 6,000 years ago. The famed burial mask of Egyptian king Tutankhamun was composed of gold. Because gold is so easy to work with, it accounts for around 78% of total gold mined each year.
- Since 700 BC, gold has been utilized in dentistry. It is chemically impermeable, easy to install, and non-allergenic, making it ideal for fillings, crowns, bridges, and dental equipment.
- Gold is also utilized in medicine in the form of salts or radioisotopes that are taken orally or by injection to treat illnesses such as severe rheumatoid arthritis and tuberculosis.
- The gold isotope gold-198 is utilized in cancer treatment.
- Gold is used to lubricate numerous mechanical parts, conduct electricity in circuitry, and coat the inside of spaceships to shield passengers from infrared radiation and heat.
- Gold is a stable, high-efficiency conductor and connector that does not corrode, making it ideal for use in electronics. A trace quantity can be found in practically all electronic equipment, such as cell phones, televisions, calculators, and global positioning system (GPS) units.









 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...
 CUET PG Crash Course 2026: Subject-Wise ...
CUET PG Crash Course 2026: Subject-Wise ...














