Table of Contents
Grignard Reagent: Grignard reagents are the popular name for organomagnesium halides. While the reagent is particularly reactive to oxygen and moisture, Grignard reagents are frequently produced by reacting an organohalogen with magnesium in a nitrogen atmosphere. Grignard reagent, any of a number of organic magnesium (Mg) compounds given by the generic formula RMgX, where R is a hydrocarbon radical: CH3, C2H5, C6H5, and so on; and X is a halogen atom, typically chlorine, bromine, or iodine. Grignard Reagents are highly nucleophilic and have the ability to generate new carbon-carbon bonds.
Grignard Reagent
What is Grignard Reagent? Haloalkanes and additional compounds containing a halogen atom bound to either sp3- or sp2-hybridized carbon atoms, i.e. aryl and vinyl halides. Then it reacts with magnesium metal to produce organomagnesium halides, also known as Grignard reagents.
Grignard reagents are named after the French scientist Victor Grignard, who discovered them. Victor Grignard shared the Nobel Prize in Chemistry in 1912 for this accomplishment.
Typically, Grignard reagents are produced in diethyl ether (CH3CH2OCH2CH3). The reaction requires an ether solvent. The rates of reactivity of organohalogens with magnesium vary substantially. Alkyl iodides, for example, often react quite quickly, but most aryl chlorides interact very slowly.
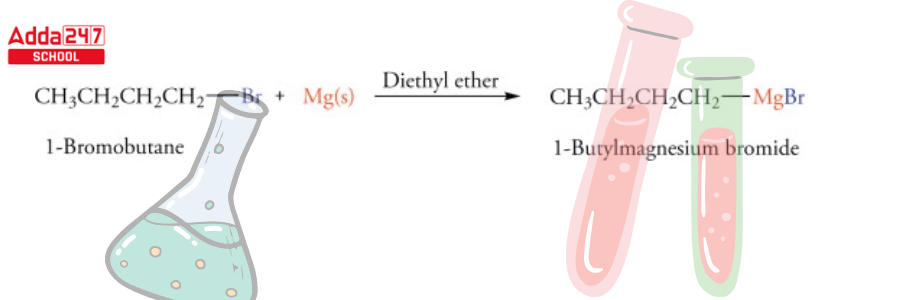
Grignard Reagent Formula
Grignard Reagents are often made by reacting an aryl or alkyl halide with magnesium. A Grignard reagent formula is ‘R-Mg-X’, where R is an alkyl or aryl group and X is a halogen.
Grignard reagents are created by combining magnesium metal with alkyl or alkenyl. As a result, The halide might be Cl, Br, or I (rather than F). Making Grignard from iodides and bromides is a difficult process. In this case, the magnesium is “inserting” itself between the carbon and the halide. Methyl magnesium bromide – CH3 MgBr and phenyl magnesium chloride – PhMgCl are two examples.
Grignard Reagent Preparation Mechanism
Several Grignard reagents can be acquired commercially, but we can also manufacture all of them in the lab. These reagents are made by treating magnesium using organic halides like alkyl or aryl halides. The following sections outline the procedure for preparing Grignard reagents.
Step 1 – Preparation of Grignard Reagent is accomplished with the assistance of ether-based solvents defined by the formula R-O-R’, since the ligands given by these solvents aid in the stabilization of the organomagnesium molecule. Grignard reagents are made by stimulating magnesium (Rieke magnesium) using organic halides in anhydrous solvents such as Diethyl ether, Et2O, or Tetrahydrofuran (THF).
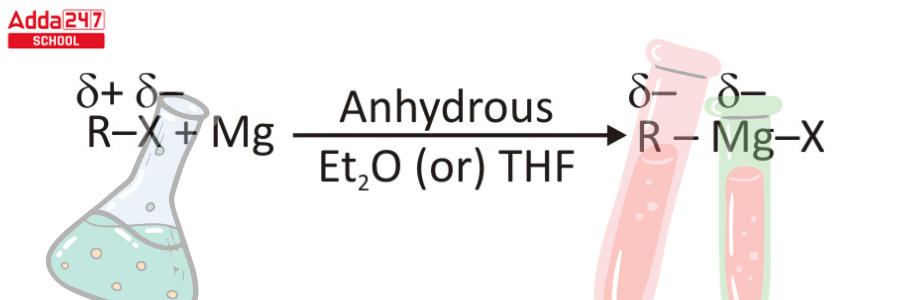
Step 2 – Grignard reagents constitute the oxidative insertion of magnesium in the center of a carbon and halogen bond, resulting in Mg(0) to Mg(II) oxidation. As a result, the mechanism underlying this reaction is not totally convincing.
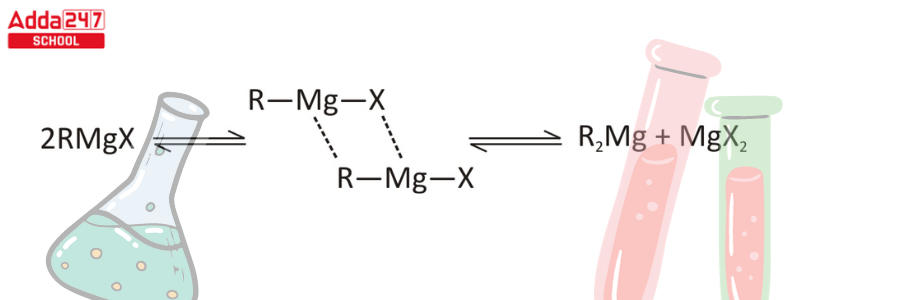
Step 3 – Grignard reagents have symmetry with R2Mg and MgX2 dialkyl magnesium species (Schlenk equilibrium).
Step 4 – The antagonism of carbon linked to the halide group reverses during the production phase of the Grignard reagent. The polar opposite is known as umpolung.
Step 5 – The process can be quite exothermic considering the reaction’s slow installation duration. As a consequence, when industrially generating the Grignard reagent, this is an important element to consider.
Grignard Reagent Formation
Grignard reagents are referred to as “RMgX.” The Grignard reagents are made by heating haloalkane and magnesium in diethyl ether (ethoxyethane). Simultaneously, this kind of reaction must be kept dry in order to prevent the resultant Grignard reagent from reacting with water. Let’s discuss the formation of Grignard Reagent now.
- The organic halide carbon atom that is linked to the halogen/halide is electrophilic. By converting to an organomagnesium halide, a Grignard reagent, this electrophilic activity can be converted to nucleophilic reactivity.
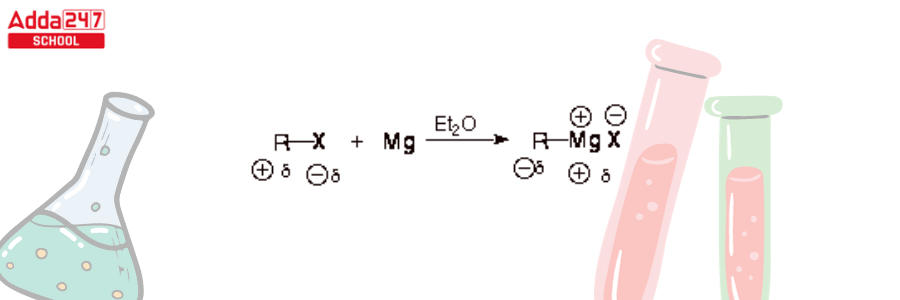
- It is significant that the carbon-magnesium bonding in a Grignard reagent is polar covalent, with carbon at the negative end of the dipole. As a result, carbon has nucleophilicity in a Grignard reagent. Also, as indicated in the arrangement above, the magnesium-halogen bond is predominantly ionic.
- It involves radical intermediates. There is one significant distinction, however. Grignard reagent creation does not require a radical chain process. It is not a chain reaction. The mechanism of creation of a Grignard reagent is depicted here.
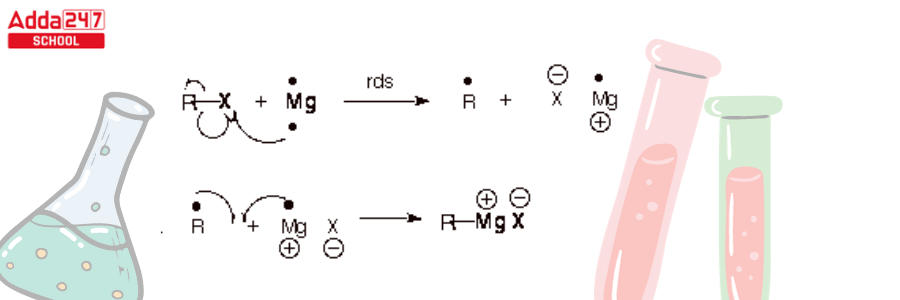
- The first step is rate-determining and includes the exchange of one electron from Mg (which has a pair of electrons in its valence shell) to the carbon-halogen bond. This produces Mg+1, which is a radical. This then reacts with the newly produced alkyl radical.
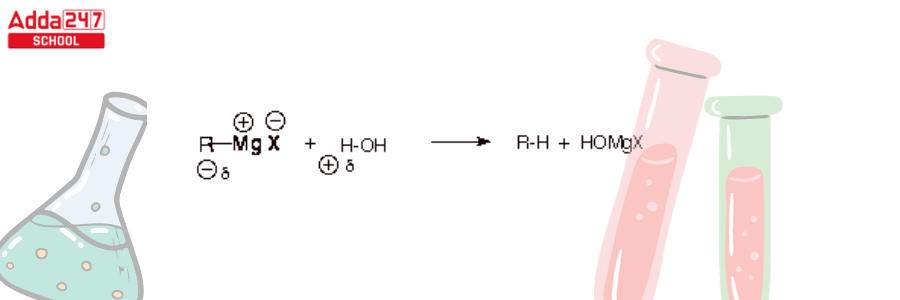
- Because ethers are non-acidic (aprotic), diethyl ether is an excellent solvent for the preparation of Grignard reagents. Since the Grignard carbon is highly nucleophilic, it would protonate and therefore disintegrate the Grignard reagent. This would result in the formation of a hydrocarbon. Grignard reagents, on the other hand, are persistent in ethers.
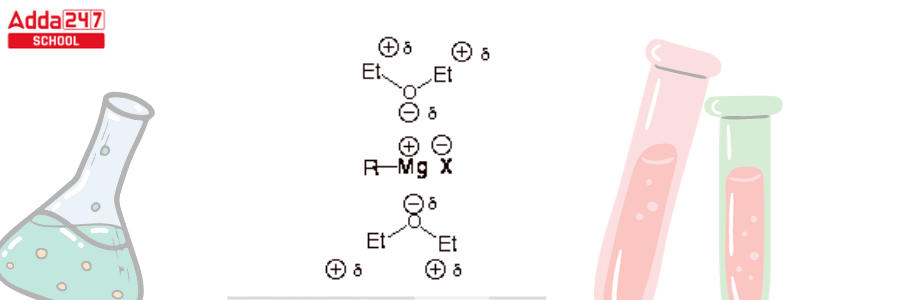
- Ethers also work well as Grignard reagent solvents because the MgX bond is ionic and advantages considerably from being well solvated. It is extremely difficult to generate ions in very nonpolar liquids where they cannot be properly solvated. Ethers are remarkably good at solvating cations as the C-O bond is comparatively polar, allowing the oxygen end of the ether dipole to solvate and stabilize the magnesium ion (electrostatically).
Reactions using Grignard Reagent
The Grignard reagent reactions are organic and uses an organomagnesium molecule, commonly known as an electrophilic “Grignard reagent,” to trigger an acidic reaction to yield a range of products. As the oxygen in these solvents stabilizes the magnesium reagent, the bulk of Grignard reactions take place in anhydrous diethyl ether or tetrahydrofuran. There must be no water present, as this could lead the reagent to degrade quickly. Let’s go over each of the Grignard reagent reactions one by one.
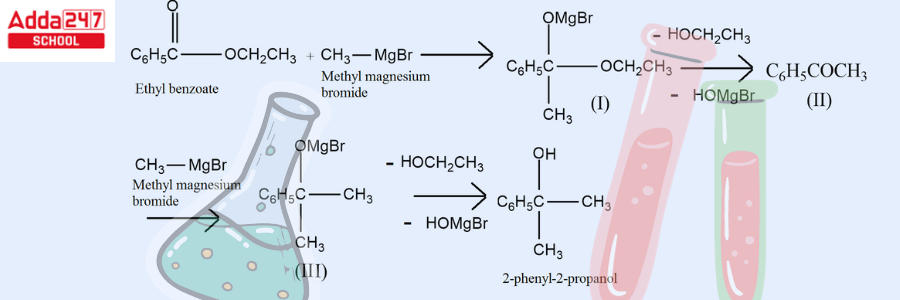
- Grignard reaction with carbonyl groups –
Since Grignard reagents are so versatile, they can produce a wide range of products when combined with carbonyl compounds. Grignard reagent reaction involves the alkylation of ketones and aldehydes with R-Mg-X in the response.
- Grignard Reagent reactions with Organic Halides –
Grignard reagents are typically quite inactive towards organic halides, in stark contrast to their reactivity towards other halides. When a metal catalyst is present, carbon-carbon coupling reactions happen with Grignard reagents serving as a reactant.In the presence of the catalyst Tris(acetylaceto) iron(III), a coupling reaction between methyl p-chlorobenzoate and nonyl magnesium bromide generates the chemical p-nonyl benzoic acid.
- Grignard Reagent reactions with Non-Carbon Electrophiles –
Grignard reagents and certain organolithium compounds are highly beneficial for the creation of fresh carbon-heteroatom bonds. These chemicals can also be transmetallated with cadmium chloride to generate dialkyl cadmium. With the assistance of these chemicals, alkyl chains can be connected to a wide range of metals and metalloids. This reaction can be expressed as follows.
2R-Mg-X + CdCl2 → R2Cd + 2Mg(X)Cl
- Industrial Reactions using Grignard Reagent-
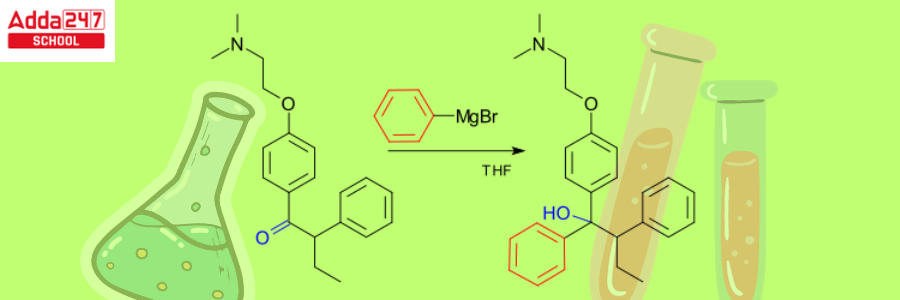
For the manufacturing process of Tamoxifen, a new type of drug that saves lives and cures breast cancer. The Grignard reagent is essential in the non-stereoselective procedure.
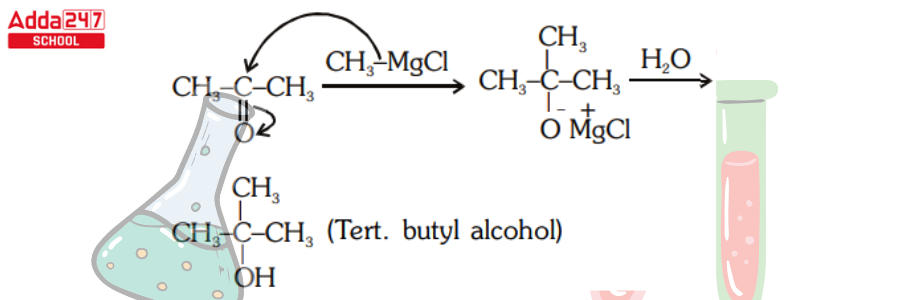
- Acetone and Methyl Magnesium Chloride Reaction –
Tertiary alcohol can be generated by reacting methyl magnesium bromide with acetone and then hydrolyzing it. Acetone cooperates with methyl magnesium bromide, which is then hydrolyzed into creating secondary alcohols.



 NEET UG 2025: Is NEET Previous years Que...
NEET UG 2025: Is NEET Previous years Que...
 JEE Mains Session 2 Result 2025 OUT, Sco...
JEE Mains Session 2 Result 2025 OUT, Sco...
 TS Inter Results 2025 Date for TSBIE 1st...
TS Inter Results 2025 Date for TSBIE 1st...










