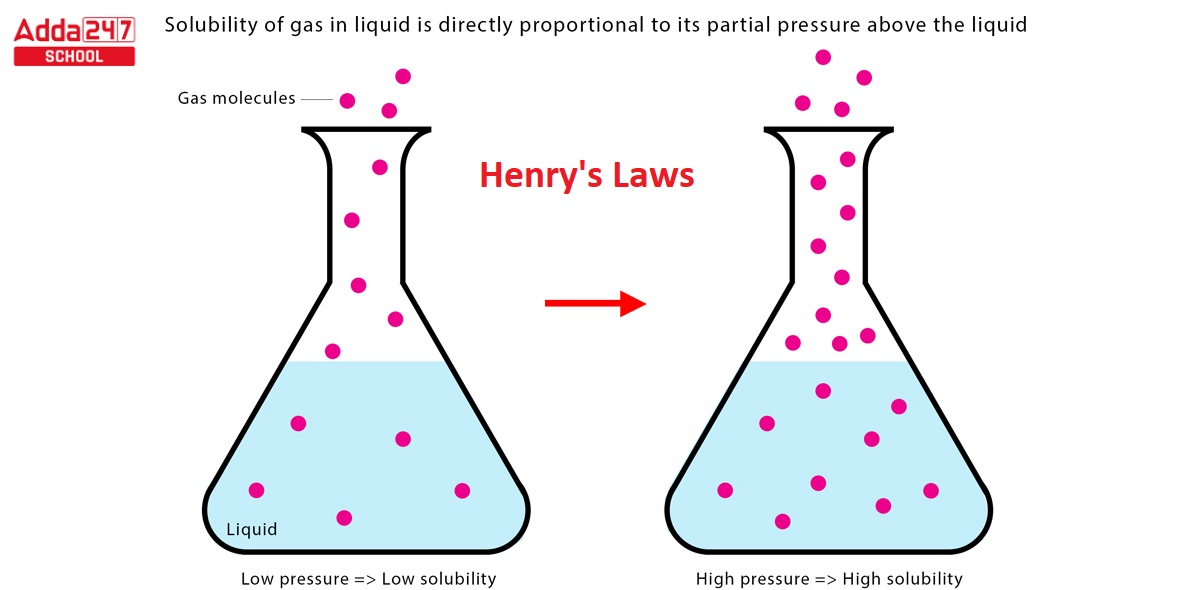
Henry’s law: According to Henry’s law, the amount of gas that dissolves in a liquid is proportional to the partial pressure of the gas above the liquid. William Henry introduced the law for the first time in 1803. In other words, the higher the partial pressure of a gas above a liquid, the more the gas dissolves in the liquid. Henry’s law is utilised in a variety of contexts, including Carbonated beverage manufacturing: Dissolving carbon dioxide gas in water produces carbonated beverages. Under high pressure, the carbon dioxide gas is dissolved, and when the pressure is removed, the carbon dioxide bubbles out of the solution.
Water purification: Henry’s law can be applied to remove dissolved gases from water. Chlorine gas, for example, can be used to eliminate dissolved oxygen from water. Food preservation: Henry’s law can be used to preserve food by inhibiting bacterial development. Carbon dioxide gas, for example, can be used to preserve food by displacing oxygen, which is required for bacteria to flourish. Underwater divers’ breathing: Divers breathe compressed air, which has a higher partial pressure of oxygen than sea-level air. Divers may now breathe underwater for longer periods of time.
Henry’s Law Statement
When the temperature remains constant, Henry’s law states that the amount of gas dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid. Henry’s law constant (typically represented by ‘kH’) is the proportionality constant for this connection. The mathematical formula for Henry’s law is as follows:
P C (or P = kH.C)
Where,
The letter ‘P’ stands for the partial pressure of the gas in the atmosphere above the liquid.
The letter ‘C’ represents the dissolved gas concentration.
‘kH’ is the gas’s Henry’s law constant.
Henry’s Law Definition
According to Henry’s law, the amount of gas that dissolves in a liquid is proportional to the partial pressure of the gas above the liquid. William Henry introduced the law for the first time in 1803. In other words, the higher the partial pressure of a gas above a liquid, the more the gas dissolves in the liquid. Carbon dioxide, for example, has a partial pressure of around 0.04% in the air. This means that at typical temperature and pressure, water contains roughly 0.04% carbon dioxide dissolved.
However, increasing the partial pressure of carbon dioxide, such as by opening a bottle of soda, causes additional carbon dioxide to dissolve in the water. When you open a can of soda, it fizzes.
Henry’s Law Examples
Here are some Henry’s Law Examples in action:
- The process of producing carbonated beverages: Carbonated beverages are produced by dissolving carbon dioxide gas in water. Under high pressure, the carbon dioxide gas is dissolved, and when the pressure is removed, the carbon dioxide bubbles out of the solution.
- Water purification: Henry’s law can be applied to remove dissolved gases from water. Chlorine gas, for example, can be used to eliminate dissolved oxygen from water.
- Food preservation: Henry’s law can be used to preserve food by inhibiting bacterial development. Carbon dioxide gas, for example, can be used to preserve food by displacing oxygen, which is required for bacteria to flourish.
- Underwater divers’ breathing: Divers breathe compressed air, which has a higher partial pressure of oxygen than sea-level air. Divers may now breathe underwater for longer periods of time.
Altitude sickness treatment: Altitude sickness is caused by the reduced partial pressure of oxygen at high elevations. By inhaling supplemental oxygen and increasing the partial pressure of oxygen in the blood, Henry’s law can be utilised to treat altitude sickness. - Coffee decaffeination: Decaffeinated coffee is produced by eliminating caffeine from coffee beans. Caffeine is a gas, and by bubbling hot water through the beans, Henry’s law can be utilised to extract caffeine from coffee beans.
- Metal extraction from ores: By bubbling a gas through the ore, Henry’s law can be used to extract metals from ores. The metal will dissolve in the gas, which can then be collected and used to remove the metal.
- Beer production: Beer is produced by fermenting malted barley with yeast. Yeast emits carbon dioxide gas, and Henry’s law can be used to regulate the amount of carbon dioxide in beer.
- Nitrogen fertiliser production: Nitrogen fertiliser is produced by mixing nitrogen and hydrogen gases. Henry’s law can be used to limit how much nitrogen dissolves in hydrogen gas.
The manufacture of oxygen for medical purposes: By bubbling air through water, Henry’s law can be used to produce oxygen for medical use. The oxygen will dissolve in the water, at which point the water can be collected and the oxygen recovered.
Henry’s Law Constant
Henry’s Law Constant is a physical constant that connects the partial pressure of a gas in a liquid to the concentration of the dissolved gas in the liquid. It is generally represented as KH or K_H. The greater the KH value, the more soluble the gas in the liquid.
Henry’s Law can be expressed mathematically as follows:
C = KH P, where:
C denotes the dissolved gas content in the liquid in moles per litre (mol/L).
P represents the partial pressure of the gas above the liquid in atmospheres (atm).
Henry’s Law is abbreviated as KH. Constant in atm mol/L units
Henry’s Law Limitations
Several factors can influence the Henry’s Law Constant, including:
- The properties of the gas and liquid: The Law of Henry A constant for a specific gas or liquid is a fixed value. However, depending on the purity of the gas and liquid, the value of this constant can vary slightly.
- Henry’s Law of Temperature With increasing temperature, the constant for a given gas or liquid often decreases. Because the kinetic energy of the gas molecules increases with temperature, they are more likely to escape from the liquid.
- The pressure: The Henry’s Law Constant for a certain gas or liquid normally increases as pressure increases. Because of the increasing pressure, the gas molecules are forced into the liquid. Henry’s Law Constant, on the other hand, does not increase forever with increasing pressure. The gas molecules can become so compressed at high pressures that they can no longer dissolve in the liquid.
Henry’s Laws Derivation
Henry’s Law describes the relationship between the concentration of a gas dissolved in a liquid and the pressure of that gas above the liquid. It states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas. Mathematically, Henry’s Law can be expressed as:
C = k * P
Where: C is the concentration of the gas in the liquid (usually in mol/L or Molarity). P is the partial pressure of the gas above the liquid (usually in atm or Pa). k is the Henry’s Law constant, which is specific for each gas-solvent pair at a given temperature.
Now, let’s derive Henry’s Law:
Assumptions:
- The gas is an ideal gas.
- The solute concentration in the liquid is relatively low, so the gas does not significantly affect the vapor pressure of the liquid.
Derivation:
- Start with the ideal gas equation:
PV = nRT
Where: P is the pressure of the gas. V is the volume of the gas. n is the number of moles of gas. R is the ideal gas constant. T is the absolute temperature in Kelvin.
- Rearrange the equation to express the number of moles (n) of the gas:
n = PV / RT
- Let’s consider a situation where a gas is in contact with a liquid, and the gas is only slightly soluble in the liquid. At equilibrium, the gas molecules will exert a partial pressure (P) above the liquid surface, and a fraction of these gas molecules will dissolve in the liquid, leading to the formation of a solution.
- Assume that the number of moles of gas dissolved in the liquid is proportional to its concentration (C) and the volume of the liquid (V_l):
n = C * V_l
- Combining equations from step 2 and step 4:
C * V_l = PV / RT
- Rearrange the equation to solve for the concentration (C):
C = PV / (V_l * RT)
- Now, introduce Henry’s Law constant (k) to account for the specific gas-solvent pair:
C = k * P
where k = 1 / (V_l * RT)
This gives us Henry’s Law:
C = k * P
where C is the concentration of the gas in the liquid, P is the partial pressure of the gas above the liquid, k is the Henry’s Law constant, which is specific for each gas-solvent pair at a given temperature, V_l is the molar volume of the liquid solvent, R is the ideal gas constant, and T is the absolute temperature in Kelvin.
It’s important to note that Henry’s Law is most accurate for gases that only slightly dissolve in liquids and at relatively low concentrations. At higher concentrations or when the gas significantly affects the vapor pressure of the liquid, deviations from Henry’s Law can occur.








 CBSE Admit Card 2026 for Private & R...
CBSE Admit Card 2026 for Private & R...
 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...














