The chemical process involves the reaction between two or more than two atoms. In chemistry, hybridization is the idea of combining two atomic orbitals to create a brand-new category of hybridized orbitals. Typically, this mixing creates hybrid orbitals with completely distinct energies, morphologies, etc.
Hybridization
In hybridization or orbital hybridisation, the same-energy level atomic orbitals are primarily involved. However, assuming they contain equal energy, both fully-filled and partially-filled orbitals can participate in this process. On the other side, we may claim that the idea of orbital hybridisation is a development of the valence bond theory and that it aids in our understanding of how bonds form as well as bond energies and lengths. Chemistry’s fascinating and vital subject of hybridization enables us to understand molecules’ forms and structures and opens new avenues for scientific research in a variety of disciplines.
What is Hybridization in Chemistry
When two atomic orbitals join to generate a hybrid orbital in a molecule, the energy of the individual atoms’ orbitals is redistributed to give orbitals of equivalent energy. We refer to this process as hybridisation. The atomic orbitals with equivalent energies are mixed together during the orbital hybridisation process, which primarily involves the fusion of two s orbitals, two p orbitals, or the mixing of a s orbital with a p orbital or a d orbital. Hybrid orbitals are the new orbitals that are created in this way. More importantly, hybrid orbitals are very helpful in describing the characteristics of atomic bonds and molecular geometry.
Types of Hybridization
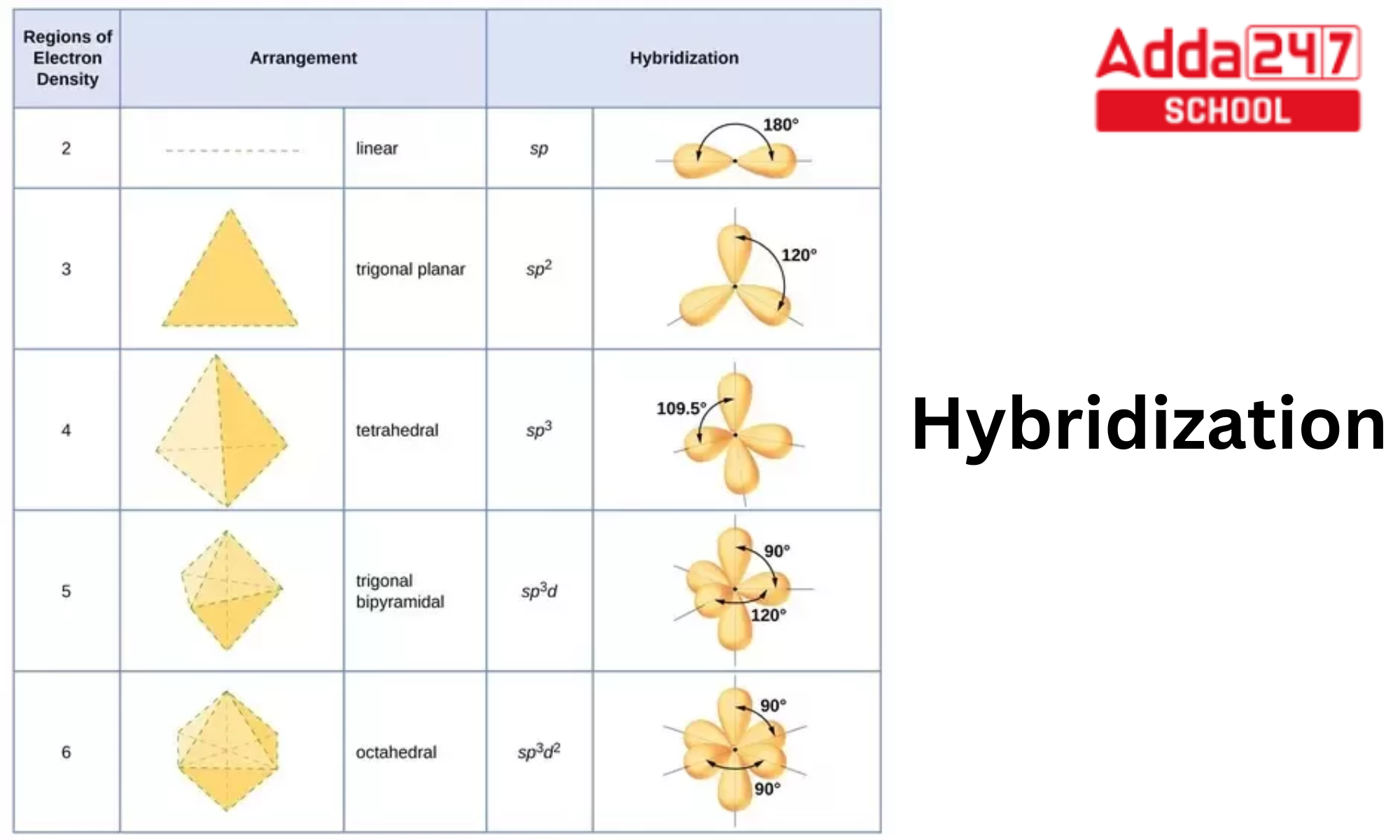
The orbital hybridisation can be categorized as sp3, sp2, sp, sp3d, sp3d2, or sp3d3 depending on the types of orbitals involved in mixing.
Dielectric Constant Definition, Formula, Meaning In Chemistry For Class 12
sp sp2 sp3 Hybridization
sp, sp2, sp3 Hybridization is explained with the help of examples.
sp Hybridisation: In sp hybridisation, one s orbital and one p orbital from the same energy level combine to form two sp hybrid orbitals. This type of hybridisation is often observed in molecules like linear ones, such as acetylene (C2H2), where carbon atoms are bonded together with a triple bond.
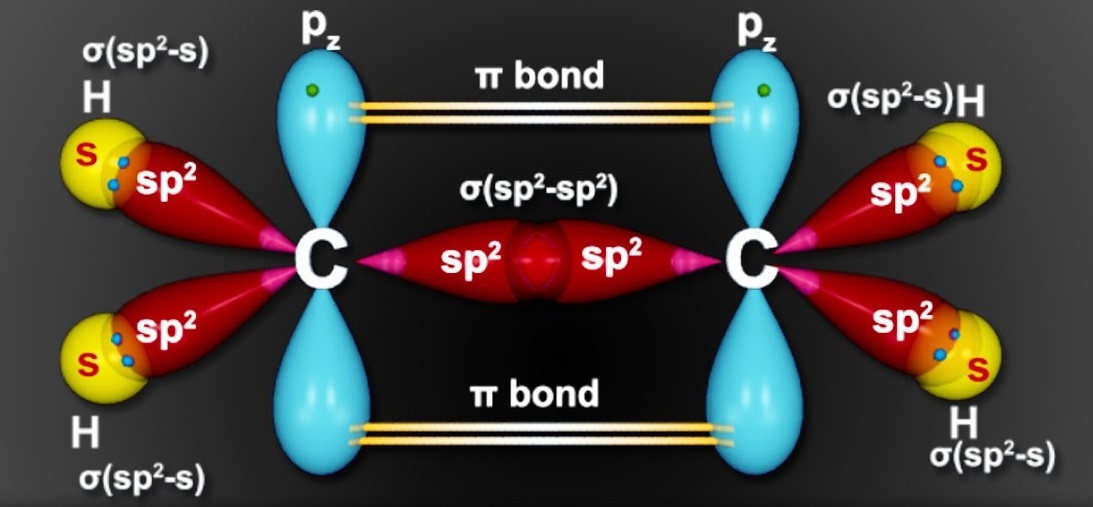
sp2 Hybridisation: In sp2 hybridisation, one s orbital and two p orbitals from the same energy level combine to form three sp2 hybrid orbitals. This type of hybridisation is commonly found in trigonal planar molecules, such as in molecules like ethene (C2H4) and boron trifluoride (BF3).
sp3 Hybridisation: In sp3 hybridisation, one s orbital and three p orbitals from the same energy level combine to form four sp3 hybrid orbitals. This type of hybridisation is commonly seen in tetrahedral molecules, such as methane (CH4) and water (H2O).
The concept of hybridisation helps to explain the observed molecular geometries and bond angles in various molecules. It’s important to note that hybridisation is a theoretical concept used to explain bonding and molecular shape, and it simplifies the complex nature of atomic orbitals in reality.
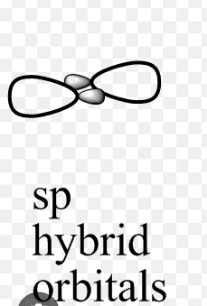
sp Hybridization
One s and one p orbital in the same main shell of an atom combine to generate two new equivalent orbitals, which is known as sp orbital hybridisation. The newly created orbitals are known as sp hybridised orbitals. It produces 180° angled linear molecules.
sp2 Hybridization
One s and two p orbitals from the same atom’s shell combine to generate three equivalent orbitals, which is known as sp2 hybridisation. Sp2 hybrid orbitals are the new orbitals that have created. Trigonal hybridisation is another name for sp2 hybridisation. To create the new hybrid orbital known as sp2, one’s’ orbital and two ‘p’ orbitals of equal energy are mixed together.
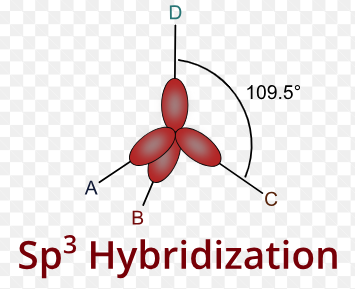
sp3 Hybridization
Tetrahedral hybridisation, or sp3, is the process by which one’s’ orbital and three ‘p’ orbitals from the same atomic shell combine to generate four new equivalent orbitals. Sp3 hybrid orbitals are the new orbitals that have generated.
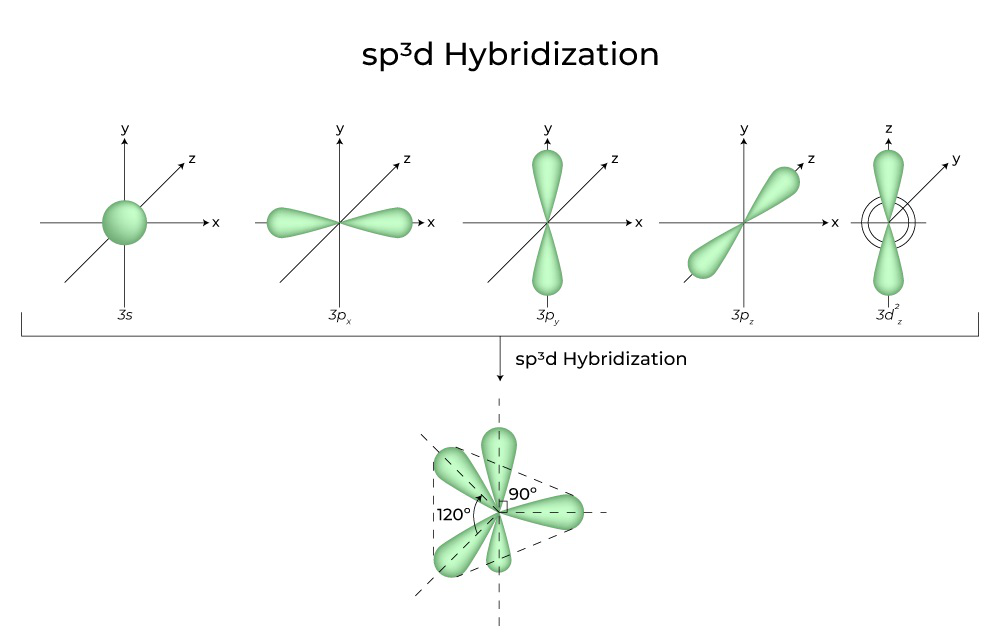
sp3d Hybridization
In sp3d hybridisation, 1s, 3p, and 1d orbitals are mixed to create 5 sp3d hybridised orbitals with identical energy. They have bipyramidal trigonal geometry. s, p, and d Orbital Synthesis Trigonal Bipyramidal Symmetry
sp3d2 Hybridization
The 1s, 3p, and 2d orbitals in sp3d2 hybridisation combine to generate six identical sp3d2 hybrid orbitals. These six orbitals are pointed in the direction of an octahedron’s four corners. They are 90 degrees apart from one another and incline.
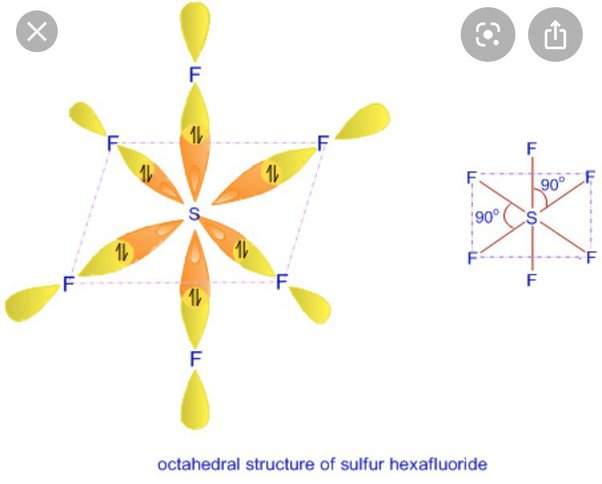
sp3d2 Hybridization Shape and Structure
sp3d2 hybridisation shape is given below in this image. look this sp3d2 hybridisation shape image and understand the shape of configration.
sp3d Hybridization Shape and Structure
sp3d Hybridisation Shape is discussed here in this given below image. Check all information related to sp3d Hybridisation Shape.
sp3 Hybridisation Shape and Stucture
sp3 hybridisation shape is given below. look sp3 hybridisation shape and understand the process.
sp2 Hybridization Shape and Stucture
Check here sp2 hybridisation shape which is given below and try to understand the process of creating sp2 hybridisation shape.
sp Hybridization Shape and Structure
Check here sp hybridisation shape which is given below.
Hybridization Formula
Here are some common hybridisation formulas for different types of atoms:
- sp Hybridisation:
- Formula: sp
- Example: Beryllium chloride (BeCl₂)
- Explanation: Beryllium undergoes sp hybridisation, where one s orbital and one p orbital combine to form two sp hybrid orbitals.
- sp² Hybridisation:
- Formula: sp²
- Example: Ethene (C₂H₄)
- Explanation: Carbon atoms in ethene undergo sp² hybridisation, where one s orbital and two p orbitals combine to form three sp² hybrid orbitals.
- sp³ Hybridisation:
- Formula: sp³
- Example: Methane (CH₄)
- Explanation: Carbon atoms in methane undergo sp³ hybridisation, where one s orbital and three p orbitals combine to form four sp³ hybrid orbitals.
- sp³d Hybridisation:
- Formula: sp³d
- Example: Phosphorus pentachloride (PCl₅)
- Explanation: Phosphorus undergoes sp³d hybridisation, where one s orbital, three p orbitals, and one d orbital combine to form five sp³d hybrid orbitals.
- sp³d² Hybridisation:
- Formula: sp³d²
- Example: Sulfur hexafluoride (SF₆)
- Explanation: Sulfur undergoes sp³d² hybridisation, where one s orbital, three p orbitals, and two d orbitals combine to form six sp³d² hybrid orbitals.
- dsp³ Hybridisation:
- Formula: dsp³
- Example: Phosphorus pentoxide (P₄O₁₀)
- Explanation: Phosphorus undergoes dsp³ hybridisation, which involves mixing one s orbital, three p orbitals, and one d orbital to form five hybrid orbitals.
sp Hybridization Examples
The sp orbital hybridisation involves the mixing of one s orbital and one p orbital to create two sp hybrid orbitals. Here are some examples of molecules and compounds that exhibit sp orbital hybridisation:
- Ethyne (Acetylene, C2H2): In ethyne, each carbon atom undergoes sp hybridisation to form two sp hybrid orbitals. These two hybrid orbitals overlap with the s orbital of hydrogen atoms to form sigma (σ) bonds. The remaining two p orbitals on each carbon atom form a pi (π) bond between them.
- Boron Trifluoride (BF3): In BF3, boron undergoes sp2 hybridisation. One of the p orbitals of boron remains unchanged, while the other two hybridize with the 2s orbital to form three sp2 hybrid orbitals. These hybrid orbitals overlap with the p orbitals of fluorine atoms, resulting in three sigma (σ) bonds.
- Beryllium Chloride (BeCl2): In BeCl2, beryllium undergoes sp hybridisation. The 2s orbital of beryllium hybridizes with one of its 2p orbitals to form two sp hybrid orbitals. These hybrid orbitals overlap with the p orbitals of two chlorine atoms to create two sigma (σ) bonds.
- Carbon Monoxide (CO): In carbon monoxide, the carbon atom undergoes sp hybridisation. One of the 2p orbitals of carbon hybridizes with its 2s orbital to form two sp hybrid orbitals. One of these hybrid orbitals overlaps with an orbital from oxygen to create a sigma (σ) bond, while the other hybrid orbital holds a lone pair.
- Formaldehyde (CH2O): In formaldehyde, the carbon atom undergoes sp2 hybridisation. One of the 2p orbitals of carbon hybridizes with its 2s orbital to form three sp2 hybrid orbitals. One of these hybrid orbitals forms a sigma (σ) bond with hydrogen, another overlaps with oxygen to create a sigma (σ) bond, and the third hybrid orbital holds a lone pair.
sp2 Hybridization Examples
some examples of molecules or compounds that exhibit sp2 hybridisation:
- Ethene (C2H4): In ethene, each carbon atom forms three sigma (σ) bonds. Two of these bonds are formed with adjacent hydrogen atoms, and the third bond is a pi (π) bond formed between the two carbon atoms. The carbon atoms in ethene are sp2 hybridized.
- Formaldehyde (CH2O): In formaldehyde, the central carbon atom forms two sigma bonds: one with two hydrogen atoms and another with an oxygen atom. The oxygen atom also has two lone pairs of electrons. The carbon atom and the oxygen atom in formaldehyde are both sp2 hybridized.
- Acetone (CH3COCH3): Acetone consists of three carbon atoms, each bonded to two other atoms (hydrogen or oxygen). The central carbon atom forms sigma bonds with two other carbon atoms and a sigma bond with a hydrogen atom. The oxygen atom is bonded to the central carbon atom via a double bond. The central carbon atom in acetone is sp2 hybridized.
- Pyridine (C5H5N): Pyridine is a heterocyclic aromatic compound. The carbon atoms in the pyridine ring are sp2 hybridized, and the lone pair of electrons on the nitrogen atom is in a p orbital, giving the molecule a planar structure.
- Benzene (C6H6): Benzene is a classic example of a compound with sp2 hybridized carbon atoms. It consists of a hexagonal ring of six carbon atoms, each bonded to two other carbon atoms and one hydrogen atom. The pi bonds in the benzene ring create a stable and planar structure.
sp3 Hybridization Examples
sp3 orbital hybridisation is a type of hybridisation that occurs when one s orbital and three p orbitals combine to form four equivalent hybrid orbitals. These hybrid orbitals are then used by atoms to form sigma bonds in molecules. Here are a few examples:
- Methane (CH4): One of the classic examples of sp3 hybridisation is methane. In methane, each carbon atom forms four sigma bonds with four hydrogen atoms. The carbon atom’s one 2s orbital and three 2p orbitals combine to form four sp3 hybrid orbitals, which are then used to bond with the four hydrogen atoms.
- Ethane (C2H6): Ethane consists of two carbon atoms and six hydrogen atoms. Each carbon atom is sp3 hybridized and forms sigma bonds with three hydrogen atoms and one carbon atom. The hybridisation of carbon in ethane allows for a tetrahedral arrangement of atoms around each carbon.
- Ammonia (NH3): In ammonia, the nitrogen atom is sp3 hybridized. It forms three sigma bonds with three hydrogen atoms and has one lone pair of electrons. The hybridisation of nitrogen allows for a pyramidal molecular geometry.
- Water (H2O): Water is another example of sp3 hybridisation. In water, the oxygen atom is sp3 hybridized, forming two sigma bonds with two hydrogen atoms and two lone pairs of electrons. The hybridisation of oxygen contributes to the bent molecular geometry of water.
- Tetrahedral Carbon in Organic Molecules: In many organic molecules, carbon atoms that are bonded to four other atoms adopt sp3 hybridisation. This allows for a tetrahedral arrangement of atoms around the carbon atom. Various alkanes, like propane, butane, and pentane, contain such sp3 hybridized carbon atoms.
- Amino Acids in Proteins: In the amino acids that make up proteins, the central carbon atom, known as the alpha carbon, is often sp3 hybridized. This hybridisation allows for the attachment of various functional groups while maintaining a tetrahedral geometry around the alpha carbon.
What Is Corrosion Of Metal With Example In Chemistry
Orbital Hybridization Examples
- All beryllium compounds, such as BeF2, BeH2, and BeCl2, as well as all compounds with a triple bond including carbon, such as C2H2, are examples of sp hybridisation.
- sp2 hybridisation examples All of the boron compounds, such as BF3 and BH3, All carbon molecules that have a carbon-carbon double bond, such as ethylene (C2H4)
- Ethane (C2H6) and methane are two examples of sp3 hybridisation.
- Example: Phosphorus pentachloride hybridisation (PCl5)
Hybridization Table Chart

Hybridization of NH3
The pyramid-shaped ammonia molecule is sp3 hybridized.
Hybridization of CO2
In essence, carbon dioxide is a sp hybridisation type. This kind of hybridisation happens when two additional atoms, specifically carbon, are linked together.
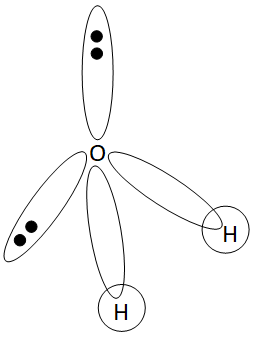
Hybridization of H2O
H2O is hybridised by sp3 hybridisation. Since the electron pairs in H2O are arranged in a tetrahedral pattern, sp3 hybridisation is necessary.
Hybridization of SO3
Trigonal planar shape and sp2 hybridisation characterize the SO3 molecule.

Hybridization of Carbon in Benzene
Carbon and hydrogen atoms are found in benzene. Sp2 type hybridisation has occurred. Each carbon atom creates two distinct bonds with two other carbon atoms that are similar during the hybridisation of benzene as opposed to just one.

Steps to Find Hybridization
One can find the orbital hybridisation of the given compound by using the basic concept of hybridisation. The steps that help in determining the hybridisation of a compound is given below.
Step 1: First, ascertain how many valence electrons an atom or ion contains overall.
Step 2: Next, determine how many lone pairs are connected to that atom or ion.
Step 3: The number of duplex or octet and lone pairs of electrons can now be added to get the number of orbitals needed.
Step 4: It should be emphasized that when there isn’t a lone pair of electrons, the geometry of orbitals in atoms or ions is different.
Hybridization Features
- Atomic orbitals with comparable energies hybridize.
- The number of atomic orbitals mixing equals the number of hybrid orbitals that are created.
- Not every orbital with half of its volume filled must participate in hybridisation. Orbitals that are fully filled and have somewhat varying energies can also take part.
- Only when a bond is formed does hybridisation take place, not when a gaseous atom is left alone.
- If the molecule’s hybridisation is known, the shape of the molecule can be predicted.
- In a hybrid orbital, the larger lobe is always positive while the smaller lobe on the other side is always negative.








 Chemistry Investigatory Project Class 12...
Chemistry Investigatory Project Class 12...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...
 CUET Chemistry Syllabus 2026, Download O...
CUET Chemistry Syllabus 2026, Download O...














