Table of Contents
The Hydrofluoric Acid Formula is essential for students to know because this acid is commonly utilized in the synthesis of several valuable fluorine compounds in the laboratory. The chemical hydrofluoric acid formula is ‘HF’. Hydrogen fluoride is a colorless, odorless gas or liquid. Hydrogen fluoride dissolves easily in water to generate colorless hydrofluoric acid solutions; dilute solutions are unrecognizable from water. It is very reactive and capable of dissolving a wide range of chemicals and compounds, including oxides. The most frequent cleaning agent is HF. Because of its acidic character, it is easier to clean materials like wheels, concrete, glass, metal, etc.
Hydrofluoric Acid Formula
We’re going to discuss the chemical formula for Hydrofluoric acid, which is HF. It is also known as fluoric acid or fluorhydric acid. The acidity of hydrofluoric acid is mostly determined by the fluoride ion’s hydrogen-bond interactions. It is a very dangerous gas in its gaseous state and highly toxic via eating and inhalation. Exposure to vapors or brief contact with liquid (hydrofluoric acid) can result in severe and painful burns. It enters the skin, causing deep-seated ulcers that can progress to gangrene. Anhydrous hydrogen fluoride (a material, particularly a crystalline compound that contains no water) appears as a colorless fume liquid boiling at 67 °F. We shall look at the Hydrofluoric acid formula, HF preparation, and its numerous properties in this article.
Chemical Formula of Hydrofluoric Acid
Hydrofluoric acid has the chemical formula – HF. Carl Wilhelm Scheele discovered it in 1771. HF is essentially a diatomic compound. Strong intermolecular hydrogen bonding exists. HF has a molar mass of 20.006 g·mol−1. Hydrofluoric acid has a density of 1.15 g/mL. The chains in the molecules are often shorter, with an average of five to six molecules.
Hydrofluoric Acid weak or strong
Hydrofluoric acid (HF) is an acid. The fact that it produces H+ in water is why it is categorized as an acid. According to the Arrhenius theory, this is a feature of an acid.
Hydrofluoric acid or HF, is a weak acid. Despite being extremely corrosive, HF is not classified as a strong acid. As the bond between H and F is so powerful HF does not dissociate easily in water. Because entirely dissociating is the key criterion of a strong acid, HF is classified as a weak acid.
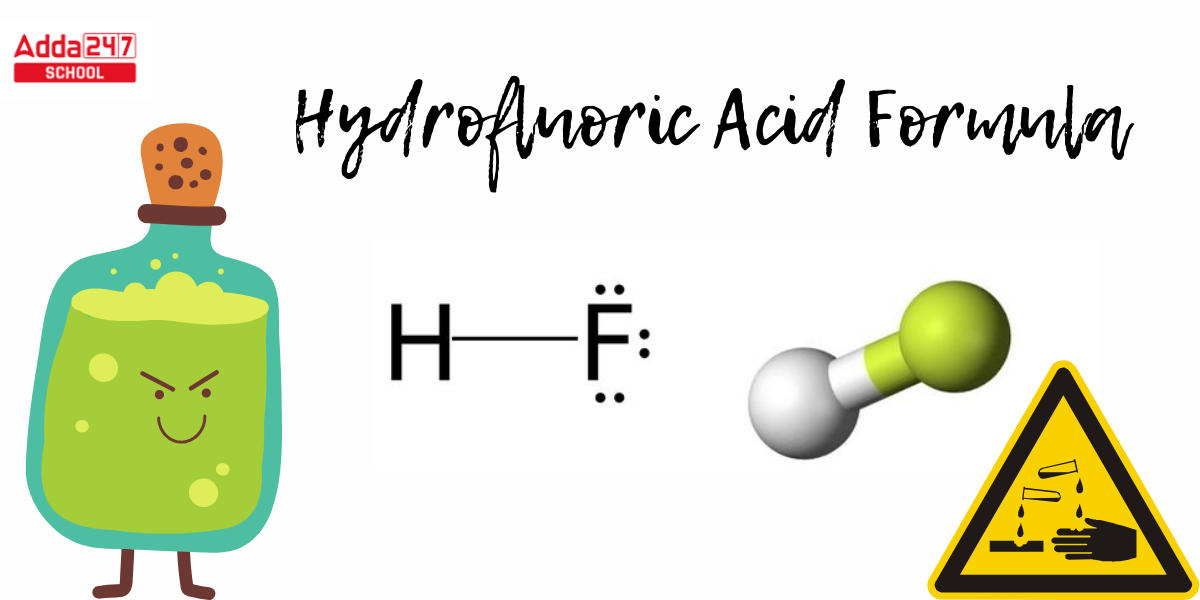
Hydrofluoric Acid Structural Formula
The Hydrofluoric Acid formula is one of the most essential and easy acid formulas.It is one of the most powerful inorganic acids.
- Fluorine and hydrogen are present in the formula for Hydrofluoric Acid. Because of the fluoride’s electronegativity, hydrogen fluoride becomes polarised. This makes it possible to simply extract the H+. As a result, it becomes an acid.
- The chemical element hydrogen has the symbol H. It has an atomic number of one and an electrical configuration of 1s1.
- Florine is a chemical element with the symbol F that is a nonmetal. It has an atomic number of 9.It has the electrical configuration 1s2 2s2 2p5.
- To create a Lewis structure for Hydrofluoric Acid. Calculate the total amount of valence electrons. Hydrogen is in the first group, with one valance electron, and fluorine has seven, for a total of eight valance electrons in the HF Lewis structure.
- Because there is no center atom here, we will just write H and F. Then we wrap two electrons between the atoms that form the chemical bond. The Hydrofluoric Acid structural formula is seen below.
Preparation of HF
The process of hydrofluoric acid preparation industrially has described below.
- To manufacture hydrofluoric acid, fluorite (CaF2) must be combined with highly concentrated sulfuric acid at an extremely elevated temperature (265 °C). This reaction produces hydrogen fluoride and calcium sulfate, as shown below:
CaF2 + H2SO4 → 2 HF + CaSO4
- Then, we mix hydrogen fluoride gas in water to produce hydrofluoric acid (HF) in varied quantities.
Physical Properties of Hydrofluoric Acid
The physical properties of Hydrofluoric Acid are listed below.
- The hydrofluoric acid solution is a colorless solution.
- At normal temperature, it has a density of 1.15 g/L, while below 20°C, it remains a colorless liquid with a density of 0.99 g/mL.
- It has a melting point of -83.6oC.
- Its boiling point is 19.5 degrees Celsius.
- however, its density, melting point, and boiling point vary due to the proportion of HF in the aqueous solution.
Hydrofluoric Acid Chemical Properties
Hydrofluoric acid is a powerful acid that is also extremely unstable and corrosive. It reacts actively with acids, oxidants, and bases. HF’s chemical characteristics are listed below.
- Silicon tetrafluoride and water are formed when silicon combines with hydrofluoric acid.
Si + 4 HF → 2 H2 + SiF4 - When hydrofluoric acid interacts with silicon dioxide, silicon tetrafluoride and water are formed.
SiO2 + 4 HF → 2 H2O + SiF4
- When hydrofluoric acid interacts with potassium, potassium fluoride is formed.
2 K + 2 HF → H2 + 2 KF - When sodium hydroxide combines with hydrofluoric acid, sodium fluoride and water are formed.
NaOH + HF —> H2O +NaF - Magnesium fluoride is formed when magnesium combines with hydrofluoric acid.
Mg + 2 HF —> H2 + MgF2
Hydrofluoric Acid Effects on Health
- Hydrofluoric acid is an extremely harmful liquid.
- HF should not be consumed, inhaled, or come into contact with the skin as it will cause severe poisoning.
Eye exposure may result in lifelong vision impairments. - Hydrofluoric acid has the ability to quickly permeate tissues while also irreversibly damaging the eyes, lungs, and mucous membranes.
- When it comes into touch with the skin, it causes severe burns. Not only that, but one may also have cardiac arrest and death.
- Even if you are only slightly exposed to it, you may get eye and throat irritation, skin burns, bone damage, and other side effects.
Hydrofluoric Acid Uses
Herbicides and medicines are made with hydrofluoric acid. More uses of the Hydrofluoric acid formula are discussed here.
- Hydrofluoric acid is most commonly used in the production of valuable fluorine compounds such as Teflon (PTFE plastic), Freon (refrigerant), and fluorocarbons.
- HF is a reagent used to dissolve silicates and oxides.
- The most prevalent application of hydrofluoric acid is in the preparation of helpful pharmaceuticals such as fluoxetine (Prozac).
- It’s found in electronics and fluorescent light bulbs.
- It is employed as a cleaning agent. It can also be used for industrial tasks such as glass etching, metal cleaning, and rust removal.
- HF is utilized as a component in the production of stain removers.
- It can also be used to clean silicon wafers in the semiconductor sector.


 NEET City Intimation Slip 2025 Out at ne...
NEET City Intimation Slip 2025 Out at ne...
 NEET Passing Marks Out of 720, Check NEE...
NEET Passing Marks Out of 720, Check NEE...
 NEET Total Marks- Check NEET Marks Distr...
NEET Total Marks- Check NEET Marks Distr...










