Table of Contents
Hypochlorous Acid: The chemical formula of Hypochlorous Acid is HOCl. Humans produce hypochlorous acid, which kills a wide spectrum of microorganisms and provides an inbuilt host defense in the body. It’s also known as hydrogen hypochlorite, chlorine hydroxide, and hypochloric acid. Hypochlorous Acid contains monovalent chlorine, which can act as a reducing or oxidizing agent. When chlorine breaks down in water, it produces hypochlorous acid, a weak and unstable acid. It is an unstable acid that serves as a human metabolite.
Hypochlorous Acid
In 1834, Antoine Jerome Balard, a French scientist, identified hypochlorous acid. It is a chlorine oxyacid that goes by several names, including perchloric acid and chloranol. Furthermore, due to the quick equilibrium with its predecessor, it cannot be separated from these solutions.
Myeloperoxidase (MPO)-catalyzed peroxidation of chloride ions produces hypochlorous acid (HOCl) primarily in leukocytes such as neutrophils, macrophages, and monocytes. HOCl is harmless and does this difficult task while being quite gentle on your skin. It is a conjugate acid of a hypochlorite and belongs to the class of reactive oxygen species. When it comes to battling bacteria, HOCl is said to be 100 times more effective than bleach.
Also Read, Oxalic Acid Formula, Properties, Structure & Application
Hypochlorous Acid Formula
HOCl represents the chemical formula of hypochlorous acid, while HClO represents the molecular formula. When chlorine is dissolved in water, a weak acid called hypochlorous acid is generated. HOCl has a molecular mass of 52.46 g.mol. It is very frequent in the human body. hypochlorous acid is also known as chloranol, perchloric acid, and chlorine hydroxide. They are produced by the human body’s immune system in order to combat infections. HOCl also kills a large variety of bacteria. To fight infections, the immune cells in the human body create hypochlorous acid.
Hypochlorous Acid Structure
Hypochlorous Acid has a simple structure. It has the chemical formula HOCl and the molecular formula HClO. The oxygen atom in the center of this acid forms a single bond with the chlorine and hydrogen atoms. Chlorine (Cl) is a halogen element and the second lightest element in Periodic Group 17 (Group VIIa). The nonmetallic chemical element oxygen (O) belongs to periodic table group 16 (VIa or oxygen group). The lightest element is hydrogen. Under normal conditions, hydrogen exists as a gas in the form of a diatomic molecule with the formula H2. Furthermore, we are able to compose its chemical structure in the usual format, as illustrated in the diagram below.
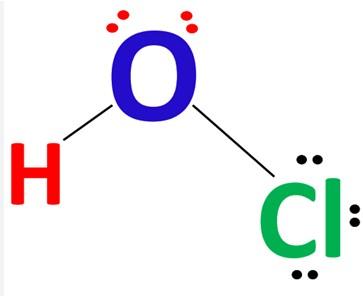
HOCl Lewis Structure
Check out, Acid Rain – Definition, Types, Causes, Effects, Examples
Preparation of Hypochlorous Acid
- Chlorine makes hypochlorous acid (HCl) and hydrochloric acid (HCl) when added to water. The reaction is as follows:
Cl2+H2OHOCl+HCl
- The process is in equilibrium, and isolating HOCl from this mixture is difficult. However, we can produce stable hypochlorous salt by combining chlorine gas with sodium hydroxide or other aqueous basic solutions.
- When acids are incorporated into aqueous salts of hypochlorous acid (such as sodium hypochlorite in a retail bleach solution), the reaction is pushed to the left, resulting in the formation of chlorine gas. Thus, dissolving chlorine gas in basic water solutions, such as sodium hydroxide, facilitates the creation of stable hypochlorite bleaches.
- Another way to make it is to dissolve dichlorine monoxide in water.
Cl2O+H2O→2HOCl
Also Read, Difference Between Acid and Base
Hypochlorous Acid Physical Properties
Only an aqueous solution of hypochlorous acid exists. Furthermore, determining its specific physical properties is challenging because they are dependent on how concentrated the solution is.
- The solution is colorless.
- HOCl has a molecular weight of 52.457 g/mol.
- HOCl has one hydrogen bond acceptor.
- Hypochlorous Acid has a monoisotopic mass of 51.972 g/mol.
- Because the molecule is in equilibrium with its anhydride, preparing dry or anhydrous hypochlorous acid is impossible.
- HOCl has one hydrogen bond donor.
Also Read, Citric Acid Formula, Structure, Properties, Uses of C6H8O7
Hypochlorous Acid Chemical Properties
- As a weak acid, hypochlorous acid breaks down in aqueous solutions into the hypochlorite ion (OCl) and H+.
- Hypochlorous acid is a powerful oxidant that can result in explosive combinations.
- When hypochlorous acid interacts with bases, hypochlorite salts are formed.
- For example, when hypochlorous acid is combined with sodium hydroxide to generate sodium hypochlorite (NaOCl), the active ingredient in bleach.
HOCl + NaOH = NaOCl + H2O
Hypochlorous Acid Chemical Reactions
Some of the important chemical reactions that HOCL performs are explained further below.
- Under normal circumstances When contrasted with chlorine, hypochlorous acid is a more powerful oxidant.
2 HClO(aq) + 2 H+ + 2 e− ⇌ Cl2(g) + 2 H2O
In this case, E = +1.63 V. - In aqueous solutions, hypochlorous acid disintegrates partially into the anion hypochlorite ClO. The following is the reaction:
HClO ⇌ ClO− + H+
- Hypochlorites contain hypochlorous acid salts. Sodium hypochlorite (NaClO), an important ingredient in bleach, is one of the most frequent hypochlorites.
- Chloramines are formed when hypochlorous acid combines with amines.
NH3 + HClO → NH2Cl + H2O
- When hypochlorous acid interacts with hydrochloric acid (HCl), chlorine gas is produced:
HClO + HCl → H2O + Cl2
Also Read, Carbonic Acid Formula, Structure, Strength, Preparation
Hypochlorous Acid Uses
There are numerous applications for hypochlorous acid. When it involves at-home use, HOCl usually comes in the form of a spray. The cleaning product is commonly accessible in spray form. Some uses of Hypochlorous Acid are given below.
- It is a more potent oxidant than chlorine and a highly effective sanitizer. It is used to produce sodium hypochlorite (NaOCl) and calcium hypochlorite (Ca(OCl)2), which are then used to generate bleaches, disinfectants, and deodorants.
- To convert alkenes to chlorohydrins, hypochlorous acid is utilized.
- Used in cosmetics, such as infant products.
- We employ an aggressive sanitizer in swimming pools.
- Used to produce a sufficient amount of safe disinfection.
- Marine sanitation equipment is used to transform seawater into HOCl.
- In cosmetics, it is used as a skin cleansing agent.
- Used to treat a variety of infections in both pets and people.


 NEET UG 2025: Is NEET Previous years Que...
NEET UG 2025: Is NEET Previous years Que...
 JEE Mains Session 2 Result 2025 OUT, Sco...
JEE Mains Session 2 Result 2025 OUT, Sco...
 TS Inter Results 2025 Date for TSBIE 1st...
TS Inter Results 2025 Date for TSBIE 1st...










