Class 12 Chemistry Important Questions
If you are searching for CBSE Class 12 Chemistry Important Questions then you are in right place. We have given CBSE Class 12 Chemistry Important Questions on this page. To get good marks in CBSE Class 12Exam the students must solve the CBSE Class 12 Chemistry Important Questions given on this page.
Important Questions for Class 12 Chemistry
We have given chapter wise CBSE Class 12 Chemistry Important Questions on this page as per the CBSE Class 12 syllabus. We have given CBSE Class 12 Chemistry Important Questions as per the latest exam pattern of CBSE Class 12 . The students appearing in the CBSE Exam can bookmark this page to get all the latest updates from CBSE regarding CBSE Class 12 exam.
Class 12 Chemistry Important Questions-Electrochemistry
Q. Name the factor on which emf of a cell depends:
Ans: Emf of a cell depends on the following factors
- Nature of reactants.
- The concentration of the solution in two half cells.
- Temperature
Q. What are the units of molar conductivity?
Ans: cm2ohm−1mol−1cm2ohm−1mol−1 or Scm2mol−1Scm2mol−1
Q. What is the effect of temperature on molar conductivity?
Ans: Molar conductivity of an electrolyte increases with an increase in temperature.
Q. Why is it not possible to measure single electrode potential?
Ans: It is not possible to measure single electrode potential because the half cell containing a single electrode cannot exist independently, as the charge cannot flow on its own in a single electrode.
Q. Is it safe to stir AgNO3 solution with a copper spoon?
(E0Ag+/ Ag= 0.80 Volt; E0Cu+2/ Cu= 0.34 Volt) (E0Ag+/ Ag= 0.80 Volt; E0Cu+2/ Cu= 0.34 Volt)
Ans: No it is not safe because it reacts with AgNO3AgNO3 Solution ( Emf will be positive.)
Q. Why is it necessary to use salt bridges in a galvanic cell?
Ans: To complete the inner circuit and to maintain the electrical neutrality of the solution.
Q. Write Nernst equation or the general cell reaction aA+bB→cC+dDaA+bB→cC+dD
Ans: Ecell = E0cell−RTln[C]c[D]dnF[A]a[B]bEcell = E0cell−RTln[C]c[D]dnF[A]a[B]b
Q. What is the EMF of the cell when the cell reaction attains equilibrium?
Ans: Zero
Q. Define electrochemical series.
Ans: The arrangement of various electrodes in the decreasing or increasing order of their standard reduction potentials is called electrochemical series.
Q. Why in a concentrated solution, a strong electrolyte shows deviations from Debye-Huckle- Onsagar equation?
Ans: Because interionic forces of attractions are large.
Q. What is the use of Platinum foil in the hydrogen electrode?
Ans: It is used for the inflow and outflow of electrons.
Q. Express the relation between conductivity and molar conductivity of a solution held in a cell.
Answer:
Λm = KC= Conductivity Concentration
Q. What is the effect of catalyst on:
(i) Gibbs energy (ΔG) and
(ii) activation energy of a reaction?
Answer:
(i) There will be no effect of catalyst on Gibbs .energy.
(ii) The catalyst provides an alternative pathway by decreasing the activation energy of a reaction.
Q. Corrosion of motor cars is of a greater problem in winter when salts are spread on roads to melt ice and snow. Why?
Ans: A short-circuited cell is formed when two metals are brought together under the surface of an electrolyte. Corrosion is more of an issue in the winter because the car contains metal like lead and chromium, and the salt sprinkled to melt ice is NaCl, which acts as an electrolyte forming a short circuit cell.
Q. What is the electrolyte used in a dry cell?
Ans: A paste of NH4ClNH4Cl , MnO2MnO2 and Carbon.
Q. How is cell constant calculated from conductance values?
Ans: Cell Constant=specific conductanceobserved conductanceCell Constant=specific conductanceobserved conductance
Q. What flows in the internal circuit of a galvanic cell?
Ans: Ions
Q. What is meant by ‘limiting molar conductivity?
Answer:
The molar conductivity of a solution at infinite dilution is called limiting molar conductivity and is represented by the symbol Λm.
Q. What is the effect of adding a catalyst on
(a) Activation energy (Ea), and
(b) Gibbs energy (AG) of a reaction?
Answer:
(a) On adding catalyst in a reaction, the activation energy reduces and rate of reaction is fastened.
(b) A catalyst does not alter Gibbs energy (AG) of a reaction.
Class 12 Chemistry Important Questions-Chemical Kinetics
Q. For the reaction R → P, the concentration of a reactant changes from 0.03 M to 0.02M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
Ans: Average rate of reaction =−ΔRΔt Average rate of reaction =−ΔRΔt
−[R]2−[R]1t2−t1−[R]2−[R]1t2−t1
⇒−0.02−0.0325=4×10−4Mmin−1⇒−0.02−0.0325=4×10−4Mmin−1
Also, it can be expressed in seconds as:
Average rate of reaction =4×10−460=6.67×10−6 Average rate of reaction =4×10−460=6.67×10−6
Q. The rate of the chemical reaction doubles for an increase of 10 K in absolute temperature from 298 K. Calculate EaEa.
Ans:
Given
T1=298KT1=298K
T2=(298+10)KT2=(298+10)K
We also know that when the temperature is raised by 10 degrees Celsius, the reaction rate doubles. As a result, we’ll use the values k1 = kk1 = k and k2 = 2kk2 = 2k.
R=8.314JK−1mol−1R=8.314JK−1mol−1
On substitution
k2k1=Ea2.303R[T2−T1T1T2]k2k1=Ea2.303R[T2−T1T1T2]
log2kk=Ea2.303×8.314[10298×308]log2kk=Ea2.303×8.314[10298×308]
Ea=2.303×8.314×298×308×log210Ea=2.303×8.314×298×308×log210
=52897.78 J mol – 1=52897.78 J mol – 1
=52.89 kJ mol – 1=52.89 kJ mol – 1
Q. The activation energy for the reaction 2HI(g)→H2 + I2(g)2HI(g)→H2 + I2(g) is 209.5 kJ mol – 1209.5 kJ mol – 1 at 581 k. Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy.
Ans:
Ea=209.5kJ – 1=209500 J mol – 1Ea=209.5kJ – 1=209500 J mol – 1
T=581K T=581K
R=8.314 J k – 1mol – 1R=8.314 J k – 1mol – 1
The percentage of reactant molecules with energy equal to or greater than activation energy is now:
x=eEa/RTx=eEa/RT
In=−Ea/RT In=−Ea/RT
logx=−Ea2.303RT logx=−Ea2.303RT
logx=209500 J mol – 12.303×8.314×Jk – 1mol – 1×581=18.8323logx=209500 J mol – 12.303×8.314×Jk – 1mol – 1×581=18.8323
x=Antilog(−18.8323)=1.47×10−19x=Antilog(−18.8323)=1.47×10−19
Q. Define ‘rate of a reaction’.
Answer:
Rate of a reaction: Either, The change in the concentration of any one of the reactants or products per unit time is called rate of a reaction. Or, The rate of a chemical reaction is the change in the molar concentration of the species taking part in a reaction per unit time.
Q. Define ‘order of a reaction’.
Answer:
The sum of powers of the concentration of the reactants in the rate law expression is called the order of reaction.
Q. If the rate constant of a reaction is k = 3 × 10-4 s-1, then identify the order of the reaction. (Comptt. All India 2013)
Answer:
S-1 is the unit for rate constant of first order reaction.
Q. Write the unit of rate constant for a zero order reaction. (Comptt. All India 2013)
Answer:
Mol L-1 S-1 is unit of rate constant for a zero order reaction.
Q. Define ‘activation energy’ of a reaction. (All India 2011)
Answer:
The minimum extra amount of energy absorbed by the reactant molecules to form the activated complex is called activation energy.
The activation energy of the reaction decreases by the use of catalyst.
Q. Define rate of reaction. (Comptt. Delhi 2016)
Answer:
The change in concentration of reactant or product per unit time is called rate of reaction.
Q. Define rate constant (K). (Comptt. All India 2016)
Answer:
Rate constant. It is defined as the rate of reaction when the concentration of reaction is taken as unity.
Q. For a reaction R → P, half-life (t1/2) is observed to be independent of the initial concentration of reactants. What is the order of reaction? (Delhi 2017)
Answer:
The t1/2 of a first order reaction is independent of initial concentration of reactants.
Class 12 Chemistry Important Questions-Surface Chemistry
Q. Define the term ‘Tyndall effect’. (Delhi 2009)
Answer:
Tyndall effect : When a beam of light is passed through a colloidal solution and viewed perpendicular to the path of the incident light, the path of light becomes visible as a bright streak. The illuminated path is called Tyndall cone and the phenomenon is called Tyndall effect.
Q. What are lyophobic colloids? Give one example for them. (All India 2011)
Answer:
Lyophobic sols : Substances like metals, their sulphides, etc., when simply mixed with the dispersion medium do not form the colloidal sol. Their colloidal sols can only be prepared by specific methods. They are not much hydrated and are irreversible in nature. They are also called extrinsic colloids.
Example : AS2S3 sol.
Q. Define ‘peptization’. (All India 2012)
Answer:
Peptization may be defined as the process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
Q. What is meant by ‘shape selective catalysis’? (All India 2012)
Answer:
The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis.
Q. Give an example of ‘shape-selective catalyst’. (Delhi 2010)
Answer:
The catalyst reaction in which small sized molecules are absorbed in the pores and cavities of selective adsorbents like zeolites is known as shape-selective catalysis.
Q. Define ‘electrophoresis’. (Delhi 2011)
Answer:
Electrophoresis : When electric current is passed through a colloidal solution, the positively charged particles move towards cathode while negatively charged particles move towards anode where they lose their charge and get coagulated. The phenomenon is known as Electrophoresis.
Q. What is meant by ‘shape-selective catalysis’ of reactions? (All India 2011)
Answer:
The catalyst reaction in which small sized molecules are absorbed in the pores and cavities of selective adsorbents like zeolites is known as shape-selective catalysis.
Q. What is the ‘coagulation’ process? (All India 2009)
Answer:
The process of settling colloidal particles is called coagulation or precipitation of the solution.
Q. What is an emulsion? (Delhi 2010)
Answer:
Emulsion is liquid-liquid colloidal system.
Q. Out of NH3 and CO2 which gas will be adsorbed more readily on the surface of activated charcoal and why? (Comptt. Delhi 2012)
Answer:
NH3 gas will be adsorbed more readily on activated charcoal. It has higher critical temperature than CO2 and is an easily liquifiable gas. Its van der Waals forces are stronger.
Q. How can a colloidal solution and true solution of the same colour be distinguished from each other? (Comptt. Delhi 2012)
Answer:
A colloidal solution scatters a beam of light but a true solution does not.
Q. How is a sol different from an emulsion ? (Comptt. All India 2012)
Answer:
A collidal sol contains solid as the dispersed phase and liquid as the dispersion medium e.g. paint, gold sol etc.
Emulsion : A colloidal dispersion in which the dispersed- phase and the dispersion medium are immiscible liquids, is known as emulsion e.g. milk, butter etc.
Q. What is especially observed when a beam of light is passed through a colloidal solution? (All India 2013)
Answer:
Tyndall effect is observed when a beam of light is passed through a colloidal solution.
Q. To which colloidal system does milk belong? (Comptt. All India 2013)
Answer:
Milk belongs to emulsion.
Q. What is electrophoresis due to? (Comptt. All India 2013)
Answer:
Electrophoresis is due to electrical charge on the colloidal particles.
Q. Write two applications of adsorption. (Comptt. All India 2012)
Answer:
Applications of adsorption :
- In decolorisation of sugar.
- In gas masks, charcoal is used which adsorbs poisonous gases in mines.
Q. Why do true solutions not show Tyndall effect? (Comptt. All India 2012)
Answer: In true solution, the diameter of the dispersed particles is much smaller than the wavelength of the light used, hence there is no scattering of light.
Q. Of physisorption or chemisorption, which has a higher enthalpy of adsorption? (All India 2013)
Answer:
Chemisorption has higher enthalpy of adsorption than physisorption due to chemical bond formation.
Q. What is meant by the term peptization? (Comptt. All India 2013)
Answer:
Peptization may be defined as the process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
Q. Give one example each of ‘oil in water’ and ‘water in oil’ emulsion. (Delhi 2014)
Answer:
Oil in water → Milk, vanishing cream
Water in oil → Butter, cold creams.
Q. What are the dispersed phase and dispersion medium in milk? (All India 2014)
Answer:
Milk : Dispersed phase → Fat (Liquid);
Dispersion medium → Liquid
Q. What is the difference between lyophobic sol and lyophilic sol? (Comptt. Delhi 2014)
Answer:
Lyophobic sols: Substances like metals, their sulphides, etc., when simply mixed with the dispersion medium do not form the colloidal sol. Their colloidal sols can only be prepared by specific methods. They are not much hydrated and are irreversible in nature. They are also called extrinsic colloids.
Example : AS2S3 sol.
Lyophilic sols: Liquid loving colloids in which there is affinity between disperse phase and dispersion medium.
Example : Starch sol, Gum sol, Gelatin sol
Q. Give one example each of sol and gel. (Delhi 2014)
Answer:
Sol → Smoke, dust
Gel → Cheese
Q. Give one example each of lyophobic sol and lyophilic sol. (Delhi 2014)
Answer:
Lyophobic sol — Metal sulphides
Lyophilic sol — Starch
Q. What is the effect of temperature on chemisorption? (All India 2014)
Answer:
Chemisorption increases with increase of temperature.
Q. Why is adsorption always exothermic? (All India 2014)
Answer:
Adsorption is accompanied by decrease of randomness. For the process to be spontaneous,
ΔG must be negative.
Hence, according to equation ΔG = ΔH – TΔS, ΔG can be -ve only if ΔH is negative.
Q. What is a ‘shape-selective catalyst’? (Comptt. Delhi 2014)
Answer:
The catalyst reaction in which small sized molecules are absorbed in the pores and cavities of selective adsorbents like zeolites is known as shape-selective catalysis.
Q. What are emulsions? Name an emulsion in which water is a dispersed phase. (Comptt. All India 2014)
Answer:
Emulsions : An emulsion is a colloidal dispersion in which both the dispersed phase and dispersion medium are liquids.
Water in oil → Butter, cold creams.
Q. What are emulsions? Give an example. (Comptt. All India 2015)
Answer:
Emulsions : An emulsion is a colloidal dispersion in which both the dispersed phase and dispersion medium are liquids. For example, milk, cream.
Q. Why is adsorption always exothermic? (Comptt. Delhi 2016)
Answer:
Due to the force of attraction/bond formation between adsorbate and adsorbent.
Q. Why is Tyndall effect shown by colloidal solutions? (Comptt. All India 2016
Answer:
It is so due to large size of colloidal particles. In colloidal solutions particle size of dispersed phase is comparable to the wavelength of light used.
Q. What are associated colloids? Given an example. (Comptt. All India 2016)
Answer:
Colloids which act as electrolyte at low concentration and show colloidal behaviour at high concentration are called Associated colloids. Example : Soap solution, Detergents.
Q. Define dialysis. (Comptt. All India 2014)
Answer:
Dialysis : The process of separating the particles of colloids from those of crystalloids by diffusion of the mixture through a parchment or an animal membrane is known as dialysis.
Q. A delta is formed at the melting point of sea water and river water. Why? (All India 2015)
Answer:
Delta is formed at the meeting point of sea water and river water due to coagulation of colloidal clay particles.
Q. In reference to surface chemistry, define dialysis. (Comptt. Delhi 2015)
Answer:
Dialysis : The process of removing the dissolved substances from a colloidal solution by means of diffusion through a suitable membrane is called dialysis.
Q. Write one similarity between physisorption and chemisorption. (Delhi 2017)
Answer:
Both physisorption and chemisorption increase with increase in pressure. Both increase with increase in surface area.
Q. What type of colloid is formed when a liquid is dispersed in a solid? Give an example. (All India) 2017
Answer:
Gel is formed when a liquid is dispersed in a solid, e.g., Cheese, butter, etc.
Q. What type of colloid is formed when a solid is dispersed in a liquid? Give an example. (All India 2017)
Answer:
Sol is formed when a solid is dispersed in a liquid, e.g., paints.
Q. What type of colloid is formed when a gas is dispersed in a liquid? Give an example. (All India 2017)
Answer:
Foam is formed when a gas is dispersed in a liquid, e.g., Froth.
Q. Which of the following is most effective in coagulating negatively charged hydrated ferric oxide sol? (Comptt. Delhi 2017)
(i) NaN03 (ii) MgSO4 (iii) AlCl3
Answer:
AlCl3 (Aluminium chloride) is most effective in coagulating negatively charged hydrated ferric oxide sol.
Q. Which of the following is most effective in coagulating positively charged hydrated ferric oxide sol? (Comptt. Delhi) 2017
(i) NaNO3 (ii) Na2SO4 (Hi) (NH4)3PO4
Answer:
Ammonium phosphate (NH4)3PO4 is most effective in coagulating positively charged hydrated ferric oxide sol.
Q. Which of the following is most effective in coagulating positively charged methylene blue sol? (Comptt. Delhi 2017)
(i) Na3PO4 (ii) K4[Fe(CN)6] (iii) Na2SO4
Answer:
Potassium ferrocyanide K4[Fe(CN)6]
Q. What are emulsions? Give an example. (Comptt. All India 2017)
Answer:
An emulsion is a colloidal dispersion in which both the dispersed phase and dispersion medium are liquids. For example, milk, cream.
Q. Write the dispersion medium and dispersed phase in milk. (Comptt. All India 2017)
Answer:
Liquid fat is the dispersed phase and water is the dispersion medium.
Q. Write the dispersed phase and dispersion medium in butter. (Comptt. All India 2017)
Answer:
Water is the dispersed phase and oil is the dispersion medium in butter.
Class 12 Chemistry Important Questions-d-and f-Block Elements
Q. Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number. (Delhi 2017)
Answer:
Cr2O72- (dichromate ion) in which oxidation state of Cr is +6 which equal to its group number 6.
Q. What is meant by ‘lanthanoid contraction’? (Delhi 2011)
Answer:
The steady decrease in the ionic radius from La3+ to Lu3+ is termed as lanthanoid contraction.
Q. Why do transition elements show variable oxidation states? (Comptt. Delhi 2014)
Answer:
The variability of oxidation state of transition elements is due to incompletely filled d-orbitals and presence of unpaired electrons, i.e. (ns) and (n -1) d electrons have approximate equal energies.
Answer:
Answer:
Permanganate ion, i.e., MnO4– with oxidation number +7.
Class 12 Chemistry Important Questions-Coordination Compounds
Q. Give IUPAC name of ionization isomer of [Ni(NH3)3NO3]Cl. (Comptt. All India 2012)
Answer:
IUPAC name : Triammine nitrato nickel (III) chloride
Q. Write down the formula of : Tetraamineaquachloridocobalt(III) chloride. (Comptt. All India 2012)
Answer:
[Co(NH3)4(H2O)Cl]Cl2
Q. Give an example of linkage isomerism. (Delhi) 2010
Answer:
Linkage isomerism : When more than one atom in an ambidentate ligand is linked with central metal ion to form two types of complexes, then the formed isomers are called linkage isomers and the phenomenon is called linkage isomerism.
[Cr(H2O)5(NCS)]2+ Pentaaquathiocyanate chromium (III) ion
[Cr(H2O)5(NCS)]2+
Pentaaquaisothiocyanate chromium (III) ion
Q. Give an example of coordination isomerism. (Delhi 2010)
Answer:
Example : [Co(NH3)6] [Cr(CN)6] and
[Cr(NH3)6] [CO(CN)6]
Q. Indicate the types of isomerisms exhibited by the complex [Co(NH3)5 (NO2)] (NO3)2. (At. no. Co = 27) (Comptt. All India 2012)
Answer:
It shows ionisation isomerism and linkage isomerism.
Q. What type of bonding helps in stabilishing the a-helix structure of proteins? (Delhi 2013)
Answer:
a-helix formation -» Intramolecular hydrogen bonding.
Q. Which complex ion is formed when undecomposed AgBr is washed with hypo solution in photography? (Comptt. All India 2013)
Answer:
Sodium dithiosulphato argentate (I) complex is formed
![]()
Q. Give IUPAC name of the ionization isomer of [Ni(NH3)3NO3]Cl. (Comptt. All India 2013)
Answer:
IUPAC name : Triammine chlorido nickel (II) nitrate [Ni(NH3)3NO3]Cl
Q. Give two examples of ligands which form coordination compounds useful in analytical chemistry. (Comptt. All India 2013)
Answer:
Examples :
(i) EDTA (Ethylene diamine tetra-acetic acid)
(ii) Dimethyl glyoxime (DMG)
Q. Which of the following is more stable complex and why?
[Co(NH3)6]3+ and [Co(en)3]3+ (Delhi 2014)
Answer:
[Co(en)3]3+ is more stable complex than [CO(NH3)6]3+ because of chelate effect.
Q. What is the IUPAC name of the complex [Ni(NH3)6]Cl2? (Comptt. Delhi 2015)
Answer:
[Ni(NH3)6]Cl2
IUPAC name : Hexaamminenickel (II) chloride.
Q. Write the IUPAC name of the following coordination compound [NiCl4]2-. (Comptt. All India 2016)
Answer:
Tetrachloridonickelate (II) ion.
Q. Why are low spin tetrahedral complexes not formed? (Comptt. Delhi 2017)
Answer:
Law spin tetrahedral complexes are rarely observed because orbital splitting energies for tetrahedral complexes are sufficiently large for forcing pairing.
Q. Write IUPAC name of the complex [Co(NH3)4Cl(NO2)]+. (Comptt. All India 2017)
Answer:
Tetra amminechloridonitro cobalt (III) ion.
Q. Write IUPAC name of the complex: [CoCl2(en2)]+ (Comptt. All India 2017)
Answer:
Dichloridobis ethylenediamine cobalt (III) ion.
Class 12 Chemistry Important Questions-Aldehydes, Ketones and Carboxylic Acids
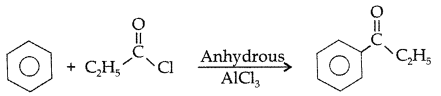
Q. Write the structure of the product formed in the following reaction : (Comptt. All India 2012)
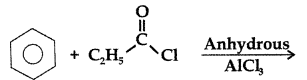
Answer:
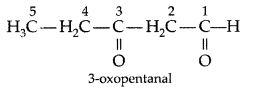
Q. Write the structure of 3-oxopentanal. (Delhi 2009)
Answer:
Q. Write the structural formula of 1-phenylpentan- 1-one. (All India 2009)
Answer:
1-Phenylpentan-1-one
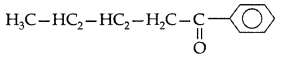
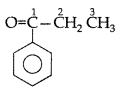
Q. Draw the structural formula of 1-phenyl propan- 1-one molecule. (Delhi 2010)
Answer:
1-phenyl propan-1-one
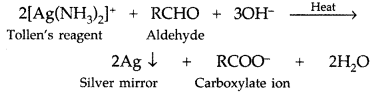
Q. What is Tollen’s reagent? Write one usefulness of this reagent. (All India 2010)
Answer:
Ammonical silver nitrate solution is called Tollen’s reagent.
Uses: It is used to test aldehydes. Both aliphatic and aromatic aldehydes reduce Tollen’s reagent to shining silver mirror. It is also used to distinguish aldehydes from ketones.
Q. Draw the structure of 3-methylbutanal. (Delhi 2011)
Answer:
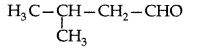
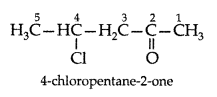
Q. Draw the structure of 4-chloropentan-2-one. (All India 2011)
Answer:
Q. Arrange the following compounds in an increasing order of their reactivity in nucleophilic addition reactions : ethanal, propanal, propanone, butanone. (Delhi 2012)
Answer:
Butanone < Propanone < Propanal < Ethanal
Q. Write the IUPAC name of the following : (All India 2012)
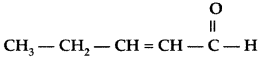
Answer:
IUPAC name : Pent-2-enal
Q. Write the IUPAC name of Ph – CH = CH – CHO. (All India 2012)
Answer:
IUPAC name : 3-phenylprop-2-enal
Q. Give a chemical test to distinguish between Benzoic acid and Phenol. (Comptt. Delhi 2012)
Answer:
Benzoic acid forms a brisk effervescence with NaHCO3 solution but phenol does not respond to this test.
Q. Give a chemical test to distinguish between Ethanal and Propanal. (Comptt. Delhi 2012)
Answer:
Ethanal on heating with I2 in NaOH gives a yellow ppt of iodoform but propanal does not respond to this test.
Q. Give a chemical test to distinguish between Propanal and Propanone. (Comptt. Delhi 2012)
Answer:
Propanone on reacting with I2 and NaOH gives a yellow ppt of iodoform but propanal does not respond to this test.
Class 12 Chemistry Important Questions-Amines
Q. Explain why
(i) The C−N−CC−N−C bond angle in trimethyl amine is 108∘108∘
Ans. The bond angle of C−N−CC−N−C is 108∘108∘ due to the repulsion between the bulky methyl groups on both the sides of the nitrogen atoms.
(ii) The quaternary ammonium salts having four different alkyl groups are optically active.
Ans. There is no rapid inversion in quaternary ammonium compounds because nitrogen atoms do not have a lone pair of electrons. The optical activity of the nitrogen atom is due to its sigma bonding to four alkyl groups.
(iii) Alkylamines are more basic than ammonia.
Ans. The alkyl group (R) pulls electrons towards nitrogen in alkyl amine due to its electron-releasing nature, making the unshared electron pair more available for sharing with the acid’s proton. Therefore, alkyl amines shows more basic nature than ammonia.
(iv) Aniline cannot be prepared by Gabriel phthalimide synthesis.
Ans. Because aryl halides cannot undergo nucleophilic substitution with the anion produced by phthalimide, Gabriel phthalimide synthesis technique cannot produce aniline.
(v) Garbriel phthalimide synthesis is preferably used for synthesising primary amines.
Ans. This process is used to make primary amines from primary alkyl halides. Because secondary and tertiary amines are not generated during this synthesis, only pure primary amines are produced, which is why this reaction is favoured for primary amine production. N-Alkyl Phthalimide is formed as a result of this reaction.
(vi) Ethylamine is soluble in water but aniline is not.
Ans. When ethylamine is mixed with water, it creates intermolecular hydrogen bonds. However, due to the presence of a large hydrophobic −C6H5−C6H5 group, aniline does not form hydrogen bond with water to a great amount. As a result, aniline is water insoluble.
(vii) Amines are soluble in dilute HClHCl .
Ans. The amines become charged when they receive an H + H + ion from the acid, allowing them to form (strong) ion-dipole interactions with water molecules. It basically turns into a salt and dissolves in the same way that table salt does in a glass of water.
(viii) Amines have lower boiling point than alcohols of comparable molecular masses.
Ans. Because nitrogen is less electronegative than oxygen, amines have lower boiling points than alcohols. As a result, the N−HN−H bond is less polar than the O−HO−H bond, and the hydrogen bond between amines and alcohols is weaker.
(ix) 1° amines have higher boiling points than 2° amines which in turn, are higher boiling than 3° amines.
Ans. Because primary amines have replacement hydrogen atoms that are accessible for hydrogen bonding, they have a higher boiling point than tertiary amines. The boiling point of tertiary amine rises due to hydrogen bonding because more heat is required to break these hydrogen bonds.
(x)The pKbpKb value of benzeneamine is 9.33 while that of ammonia is 4.75.
Ans. The pKbpKb value of benzylamine is more than ammonia because ammonia is more basic than benzyl amine. Higher pKbpKbvalue indicates lower basicity.
(xi) Aniline does not undergo Friedel-Crafts reaction.
Ans. Because the reagent AlCl3AlCl3 (the Lewis acid employed as a catalyst in Friedel crafts reactions) is electron deficient, it functions as a Lewis base and does not undergo Friedel craft reactions. It attacks the lone pair of nitrogen in aniline, forming an insoluble complex that precipitates out, stopping the process. That is why aniline does not undergo Friedal-Crafts reaction.
(xii)Aniline readily forms 2, 4, 6-tribromoaniline on reaction with bromine water.
Ans. Because of the nitrogen atom and the low + I + I impact of hydrogen, aniline is a strongly activating group. This results in a highly dense electron cloud in benzene, resulting in a strong reaction with Br water, yielding 2, 4, 6-Tribromoaniline.
(xiii) Sulphanilic acid is insoluble in water.
Ans. Because it cannot establish hydrogen bonds with water molecules, sulphanilic acid is insoluble in both water and acid. However, it is soluble in aqueous mineral acids such as HFHF , HClHCl , and HNO3HNO3 because mineral acids may form hydrogen bonds with them.
(xiv)Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
Ans. In water, methylamine interacts with ferric chloride to form ferric hydroxide. Methylamine is more basic than water due to the + I + I action of the −CH3−CH3 group. As a result, methylamine creates OH−OH− ions in water by absorbing H + H + ions from the environment. Methyl amine is a base that produces hydroxide ions when it dissolves in water.
(xv)Diazonium salt of aromatic amines are more stable than the diazonium salts of aliphatic amines.
Ans. The positive charge on the benzene ring disperses due to resonance. The diazonium ion’s stability is due to this resonance. As a result, aromatic amine diazonium salts are more stable than aliphatic amine diazonium salts.
(xvi)Although amino group is o, p-directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
Ans. Nitration takes place in an acidic environment. Aniline is protonated to yield anilinium ion in an acidic media (which is meta-directing).
Q. How can you increase the reduction potential of an electrode? For the reaction?
Mn+(aq)+ne−1→M(s)Mn+(aq)+ne−1→M(s)
Ans: Nernst equation is:
E0Mn+M=EMn+M − 2.303RTnFlog1[Mn+]E0Mn+M=EMn+M − 2.303RTnFlog1[Mn+]
EMn+MEMn+M can be increased by
- increase in concentration of Mn+Mn+ ions in solution
- By increasing the temperature.
Q. Calculate emf of the following cell at 298K298K.
Zn/Zn2+(10−4M)|| Cu2+(10−2M)/CuZn/Zn2+(10−4M)|| Cu2+(10−2M)/Cu
Given E0Zn2+/Zn =−0.76VEZn2+/Zn0 =−0.76V
E0Cu2+/Cu=+0.34V ECu2+/Cu0=+0.34V
Ans: Cell reaction is as follows.
Zn(s)+ Cu2+(aq)→ Zn2+(aq) + Cu(s) Zn(s)+ Cu2+(aq)→ Zn2+(aq) + Cu(s)
n = 2 and T = 298 K
Ecell=(E0Cu2+/Cu−E0Zn2+/Zn)−0.0591nlog [Zn2+(aq)][Cu+2(aq)]Ecell=(ECu2+/Cu0−EZn2+/Zn0)−0.0591nlog [Zn2+(aq)][Cu+2(aq)]
Ecell=0.34V−(−0.76)−0.05912log10−410−2Ecell=0.34V−(−0.76)−0.05912log10−410−2
Ecell=1.10−0.02955log10−2Ecell=1.10−0.02955log10−2
=1.10+2×0.02955=1.10+2×0.02955
∴Ecell=+1.1591V∴Ecell=+1.1591V
Q. Electrolysis of KBr(aq)KBr(aq) gives Br2Br2 at anode but KF(aq)KF(aq) does not give F2F2 . Give a reason.
Ans: Oxidation takes place at anode. Now higher the oxidation Potential, easier to oxidize. Oxidation potential of Br−Br−, H2OH2O, F−F− are in the following order. Br−>H2O>F−Br−>H2O>F−
Therefore in the Aqueous Solution of KBr. Br−Br− ions are oxidized to Br2Br2 in preference to H2OH2O . On the other hand, in the aqueous solution of KFKF, H2OH2O is oxidized in preference to F−F− . Thus in this case oxidation of H2OH2O at anode gives O2O2 and no F2F2 is produced.
Q. What happens when a piece of copper is added to An aqueous solution of FeSO4FeSO4 ?
Ans: Nothing will happen when the piece of copper is added to FeSo4FeSo4 because reduction potential E0Cu+2/Cu (−0.34V)ECu+2/Cu0 (−0.34V) is more than the reduction potential E0(Fe2+/Fe)(−0.44V)E(Fe2+/Fe)0(−0.44V) therefore copper is less reactive than iron.
Q. An aqueous solution of FeCl3FeCl3 ?
Ans: copper will dissolve in an aqaq solution of FeCl3FeCl3 because reduction potential E0(Fe3+/Fe2+)(+0.77V)E(Fe3+/Fe2+)0(+0.77V) is more than the reduction potential E0Cu+2/Cu (−0.34V)ECu+2/Cu0 (−0.34V) .
Cu(s)+ 2FeCl3 (aq) → Cu+2(aq) + 2 FeCl2(aq)Cu(s)+ 2FeCl3 (aq) → Cu+2(aq) + 2 FeCl2(aq)
Q. Define corrosion. Write the chemical formula of rust.
Ans: Corrosion is a process of deterioration of a metal as a result of its reaction with air and water surrounding it. It is due to the formulation of sulphides, oxides, carbonates, hydroxides, etc.
Formula of rust- Fe2O3.xH2OFe2O3.xH2O
Q. Write short notes on reduction and oxidation potentials.
Ans: Oxidation potential: This is a measure of the tendency of a chemical species to lose electrons or to get oxidized at an electrode.
Reduction potential: This is a measure of the tendency of a chemical species to gain electrons or to get reduced at an electrode.
Q. How are standard electrode potentials measured?
Ans: The standard electrode potential is measured by a galvanic cell which is constructed with a standard hydrogen electrode on one side and an unknown half cell on the other side.
Q. What is a cell constant? How is it determined?
Ans: Cell constant is defined as the ratio of the distance between the electrodes which is divided by the area of the cross-sectional of the electrode. Cell constant can be determined by calculating the resistance of a cell of known conductivity..
Q. What is conductivity water?
Ans: Conductivity water is a measure of the ability of water to pass an electric current.
Q. Why is it necessary to platinize the electrodes of a conductivity cell before it is used for conductance measurement?
Ans: Because platinization will increase the surface area of the electrode on which the hydrogen gas will contact with hydrogen ions. Due to this the reactions happen faster and lead to better conduction measurement.
Q. Why does mercury cells give constant voltage?
Ans: Mercury cells give constant voltage because overall cell reaction does not include any ion in the solution whose concentration changes during its lifetime.
Q. What is a fuel cell? Write reaction involved in H2−O2H2−O2 fuel cell.
Ans: An electrochemical cell in which the chemical energy of the fuel is directly converted into electrical energy is known as a Fuel cell. The Half cell reactions in Hydrogen-oxygen fuel cell as follows:
Oxidation at anode:
2H2(g)+4OH−(aq)→4H2O(l)+4e−2H2(g)+4OH−(aq)→4H2O(l)+4e−
Reduction at cathode:
O2(g)+2H2O(l)+4e−→4OH−(aq)O2(g)+2H2O(l)+4e−→4OH−(aq)
Q. Why is Li the best reducing agent whereas Fluorine is the best oxidizing agent? but not to EcellEcell . Explain.
Ans: according to the electrochemical series, Li ion has the lowest reduction potential which means it acts as the best reducing agent. Similarly, F has the highest reduction potential, thus fluorine oxidizes another substance readily. So, F is the best oxidizing agent.
Q. Why sodium metal is not obtained at cathode when aqNaClaqNaCl is electrolysed with PtPt electrodes but obtained when molten NaClNaCl is electrolysed?
Ans: Because the reduction potential of Na+Na+ , E0Na+/Na=−2.7VENa+/Na0=−2.7V is energetically more difficult than the reduction of water.
Q. Zn rod weighing 25g25g was kept in 100 mL100 mL of 1M1M copper sulphate solution. After certain time interval, the molarity of Cu2+Cu2+ was found to be 0.8 M0.8 M . What is the molarity of SO4−2SO4−2 in the resulting solution and what should be the mass of ZnZn rod after cleaning and drying?
Ans: weight of Zn rod = 25g
Number of moles that are required by Cu2+Cu2+ to Cu = Molarity×volumeMolarity×volume
=(1−0.8)×100L1000=0.2×0.1=0.02mole=(1−0.8)×100L1000=0.2×0.1=0.02mole
Molar mass of Zinc = 64.5g/mol
The zinc gets oxidized = 0.02×65.4=1.308g0.02×65.4=1.308g
Hence, the weight of Zn rod left will be = 25−1.308=23.69gm25−1.308=23.69gm
Q. Which will have greater molar conductivity and why? Sol A. 1 mol KCl dissolved in 200cc of the solution or Sol B. 1 mol KCl dissolved in 500cc of the solution.
Ans: Sol B: 1 mol KCl has dissolved in 500c of the solution has greater molar conductivity. Because the number of ions in Sol B is greater than Sol A.
Molar conductivity is the conduction of all moles produced from 1mole of KCl and which increases with dilution.
Q. Two half cell reactions of an electrochemical cell are given below :
MnO–4(aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I), E° = + 1.51 V
Sn2+ (aq) → 4 Sn4+ (aq) + 2e–, E° = + 0.15 V
Construct the redox equation from the two half cell reactions and predict if this reaction favours formation of reactants or product shown in the equation.
Answer:
The reactions can be represented at anode and at cathode in the following ways :
At anode (oxidation) :
Sn2+ → = Sn4+ (aq) + 2e– ] × 5 E° = + 0.15 V
At cathode (reduction) :
MnO–4(aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I)] × 2 E° = + 1.51 V
The Net R × M = 2MnO–4(aq) + 16H+ + 5Sn2+ → 2Mn2+ + 5Sn4+ + 8H2O
Now E°cell = E°cathode – E°anode
= 1.51 – 0.15 = + 1.36 V
∴ Positive value of E°cell favours formation of product.
Q. Express the relation among the cell constant, the resistance of the solution in the cell and the conductivity of the solution. How is the conductivity of a solution related to its molar conductivity?
Answer:
1R×1a = Conductance (C) × Cell constant
Molar conductance : (Λm) = K×1000c.
Q. Given that the standard electrode potentials (E°) of metals are :
K+/K = -2.93 V, Ag+/Ag = 0.80 V, Cu2+/Cu = 0.34 V,
Mg2+/Mg = -2.37 V, Cr3+/Cr = -0.74 V, Fe2+/Fe = -0.44 V.
Arrange these metals in increasing order of their reducing power.
Answer:
Ag+/Ag < Cu2+/Cu < Fe2+/Fe < Cr3+/Cr < Mg2+/ Mg < K+/K
More negative the value of standard electrode potentials of metals is, more will be the reducing power.
Q. Two half-reactions of an electrochemical cell are given below :
MnO–4 (aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I), E° = 1.51 V
Sn2+ (aq) → Sn4+ (aq) + 2e–, E° = + 0.15 V.
Construct the redox reaction equation from the two half-reactions and calculate the cell potential from the standard potentials and predict if the reaction is reactant or product favoured.
Answer:
The reactions can be represented at anode and at cathode in the following ways :
At anode (oxidation) :
Sn2+ → Sn4+ (aq) + 2e– ] × 5 E° = + 0.15 V
Af cathode (reduction) :
MnO–4(aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I)] × 2 E° = + 1.51 V
The Net R × M = 2MnO–4(aq) + 16H+ + 5Sn2+ → 2Mn2+ + 5Sn4+ + 8H2O
Now E°cell = E°cathode – E°anode
= 1.51 – 0.15 = + 1.36 V
∴ Positive value of E°cell favours formation of product.
Q. Write any three differences between potential difference and e.m.f.
Ans:
| E.M.F | POTENTIAL DIFFERENCE |
| It is the difference between the electrode potential of two electrodes when no current is flowing through the circuit. | It is the difference of potential between the electrodes in a closed circuit. |
| It is the maximum voltage obtained from a cell. | It is less than the maximum voltage obtained from a cell. |
| It is responsible for the steady flow of current. | It is not responsible for the steady flow of current. |
Q. Why does an electrochemical cell stop working after some time?
Ans: The reduction potential of an electrode depends upon the concentration of a solution with which it is in contact. As the cell works, the concentration of reactants decreases. Then according to Le Chatelier’s principle, it will shift the equilibrium in the backward direction. On the other hand, if the concentration is more on the reactant side then it will shift the equilibrium in the forward direction. When a cell works, concentration in an anodic compartment in the cathodic compartment decreases, and hence E0cathodeEcathode0 cathode will decrease. Now EMF of the cell is E0cell= E0cathode – E0anodeEcell0= Ecathode0 – Eanode0
A decrease inE0cathodeE0cathode and a corresponding increase in E0anodeE0anode will mean that EMF of the cell will decrease and will ultimately become zero i.e., the cell stops working after some time.
Q. Write the reactions taking place at cathode and anode in lead storage battery when the battery is in use. What happens on charging the battery ?
Answer:
At Anode: Pb + SO4-2 → PbSO4 + 2e
at Cathode : PbO2 + SO4-2 + 4H+ + 2e → PbSO4 + 2H2O
On charging the battery, the reaction is reversed and PbSO4 on anode and cathode is converted into Pb and PbO2 respectively.
Q. The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm-1. Calculate its molar conductivity.
Answer:
Molar conductivity Λm = 1000×κM
Given : K = 0.025 S cm-1, M = 0.20 M
Hence, Λm = 0.025×10000.20 ∴ Λm = 125 S cm2 mol-1
Q. The standard electrode potential (E°) for Daniel cell is +1.1 V. Calculate the ΔG° for the reaction
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
(1 F = 96500 C mol-1). (All India 2013)
Answer:
We know, ΔG° = -nF E°cell
Given : E°cell = 1.1 volt
∴ ΔG° = -2 × 96500 C mol-1 × 1.1 volt
= -212300 CV mol-1
= -212300 J mol-1 = -212.3 KJ mol-1
Q. For the standard cell,
Cu(s)/Cu+2(aq)|| Ag+(aq)/Ag(s) Cu(s)/Cu+2(aq)|| Ag+(aq)/Ag(s)
E0Cu2+/cu=+0.34VECu2+/cu0=+0.34V
E0Ag+/Ag =+0.80 V EAg+/Ag0 =+0.80 V
(i). Identify the cathode and the anode as the current is drawn from the cell.
Ans: From the cell representation, Ag+/AgAg+/Ag electrode is cathode and Cu/Cu2+Cu/Cu2+ electrode is anode.
(ii). Write the reaction taking place at the electrodes.
Ans:
At anode:
Cu(s)→Cu2+(aq)+2e−Cu(s)→Cu2+(aq)+2e−
At cathode:
2Ag+(aq)+2e−→Ag(s)2Ag+(aq)+2e−→Ag(s)
(iii). Calculate the standard cell potential.
Ans:
E0cell= E0cathode – E0anodeEcell0= Ecathode0 – Eanode0
E0cell=E0Ag+/Ag−E0Cu2+/CuEcell0=EAg+/Ag0−ECu2+/Cu0
The standard cell potential,E0cell=+0.80V−(+0.34V)=+0.46VEcell0=+0.80V−(+0.34V)=+0.46V
Question 20.
State Kohlrausch law of independent migration of ions. Why does the conductivity of a solution decrease with dilution? (All India 2014)
Answer:
Kohlrausch law of independent migration of ions: The limiting molar conductivity of an electrolyte (i.e. molar conductivity at infinite dilution) is the sum of the limiting ionic conductivities of the cation and the anion each multiplied with the number of ions present in one formula unit of the electrolyte
Λ°m for AxBy = xλ°+ + yλ°–
For acetic acid Λ° (CH3COOH) = λ°CH3COO– + λ°H+
Λ°(CH3COOH) = Λ° (CH3COOK) + Λ° (HCl) – Λ° (KCl)
Q. Define the following terms :
(i) Fuel cell
(ii) Limiting molar conductivity (Λ°m)
Answer:
(i) Fuel cells : These cells are the devices which convert the energy produced during combustion of fuels like H2, CH4, etc. directly into electrical energy.
(ii) The molar conductivity of a solution at infinite dilution is called limiting molar conductivity and is represented by the symbol Λ°m.
Q. Define the following terms :
(i) Molar conductivity (Λm)
(ii) Secondary batteries (All India 2014)
Answer:
Molar conductivity: Molar conductivity of a solution at a given concentration is the conductance of the volume ‘V’ of a solution containing one mole of electrolyte kept between two electrodes with area of cross section ‘A’ and distance of unit length. It is represented by Λm (lamda).
Λm = KAl I = 1 and A = V
∴ Λm = KV Unit = S cm2 mol-1
Secondary batteries : Those batteries which can be recharged by passing an electric current through them and can be used again and again are called secondary batteries.
Q. Can we store copper sulphate in Zinc vessels and silver vessels? Give reasons.
Given E0Cu2+/Cu = +0.34V;E0Zn2+/Zn= −0.76V; E0Ag+/Ag = +0.80V ECu2+/Cu0 = +0.34V;EZn2+/Zn0= −0.76V; EAg+/Ag0 = +0.80V
Ans: A metal having lower reduction potential can displace a metal having higher reduction potential from solution of its salt of Cu2+(E0Cu2+/Cu=+0.34V)Cu2+(ECu2+/Cu0=+0.34V) . Since standard reduction potential of Zn2+(E0Zn2+/Zn=−0.76V)Zn2+(EZn2+/Zn0=−0.76V) is less than the standard reduction potential of Cu2+(E0Cu2+/Cu=+0.34V)Cu2+(ECu2+/Cu0=+0.34V) ,ZnZn can displace copper from copper sulphate solution. Thus, CuSo4CuSo4 solution cannot be stored in a zinc vessel.
Since the reduction potential of silver is more than copper so silver is less reactive than copper and will not be able to displace copper. Hence CuSO4 solution can be stored in a silver vessel.
Q. How many grams of chlorine can be produced by the electrolysis of matters NaClNaCl with a current of 1.02A1.02A for 1515 min?
Ans: 2NaCl(l) →2Na+(l)+2Cl−(l) 2NaCl(l) →2Na+(l)+2Cl−(l)
2 Cl2mole−→Cl2(g)1mole + 2e 2 Cl2mole−→Cl2(g)1mole + 2e
Q= nfQ= nf
Q= 2 × 96500 C/mol= 1.93 × 105C Q= 2 × 96500 C/mol= 1.93 × 105C
Quantity of electricity used = I×t=1.02A×(15×60)s=900CI×t=1.02A×(15×60)s=900C
Molar mass of Cl2Cl2 = 2×35.5=71g2×35.5=71g
∴1.93×105C∴1.93×105C of charge produce chlorine = 71gm
900C of charge produce chlorine = 71×9001.93×10571×9001.93×105 =0.331gm
Hence, 0.331 grams of chlorine can be produced by the electrolysis of matter NaClNaCl with a current of 1.02 A1.02 A for 1515 min.
Q. What is understood by a normal hydrogen electrode? Give its significance.
Ans: Normal hydrogen electrode is also known as Standard Hydrogen Electrode which is used for reference on half cell potential reactions.
By using normal hydrogen electrodes, we can calculate cell potentials using different electrodes and this is a standard measurement of electrode potential for the thermodynamic scale of redox potential.
Q. Define the following terms :
(i) Rate constant (k)
(ii) Activation energy (Ea)
(i) Rate constant (k): It is a proportionality constant and is equal to the rate of reaction when the molar concentration of each of the reactants is unity.
(ii) Activation energy (Ea): The minimum extra amount of energy absorbed by the reactant molecules to form the activated complex is called activation energy.
Q. Set up Nemst equation for the standard dry cell. Using this equation show that the voltage of a dry cell has to decrease with use.
Answer:
Cell reaction of a dry cell can be represented as
Zn + Hg2+ → Zn2+ + Hg (n = 2)
Nemst equation
Ecell = E°cell – 0.05912log[Zn2+]Hg2+
The voltage of dry cell has to decrease because the concentration of electrolyte decreases in the reactions.
Q. Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their Variation with change in temperature.
Answer:
Conductivity: Conductivity of a solution is defined as the conductance of a solution of 1 cm length and having 1 sq. cm as the area of cross-section. It is represented by K.
Its unit is S cm-1
Molar conductivity : Molar conductivity of a solution at a dilution V is the conductance of all the ions produced from one mole of the electrolyte dissolved in V cm3 of the solution when the electrodes are 1 cm apart and the area of the electrodes is so large that the whole of the solution is contained between them. It is represented by Λm.
Its unit is S cm2 mol-1
Conductivity and molar conductivity of electrolytes increase with increasing temperature.
Q. Define electrode potential. Why the absolute value of the reduction potential of the electrode cannot be determined?
Ans: The voltage or potential difference of a cell assembled from a standard hydrogen electrode is known as electrode potential for a given electrode.
The potential is determined by using a standard hydrogen electrode as a reference and so absolute potential cannot.
Q. Write the equation showing the effect of concentration on the electrode potential.
Ans: The equation showing the effect of concentration on the electrode potential is known as Nernst Equation.
Ecell=E0cell−RTnFlnQEcell=Ecell0−RTnFlnQ
Where, EcellEcell = the reduction potential at current conditions
E0cellEcell0 = the standard reduction potential relative to hydrogen’s reduction potential at 250C250C
R = universal gas constant
T =temperature in K
n = the moles of electrons transferred between the positive and negative terminals of an electrochemical system.
F =faraday’s constant
Q = reaction quotient
Q. Derive the relationship between Gibbs’s free energy change and the cell potential.
Ans: The electrical work of electrical energy produced is equal to the product of e.m.f of a cell. (EcellEcell)
Welectrical=−Ecell×qWelectrical=−Ecell×q
The negative sign indicates the work done by the system on the surroundings.
Therefore, Welectrical=−nFEcellWelectrical=−nFEcell
Since, ΔG=−W(non-expansion)ΔG=−W(non-expansion)
In this case non-expansion work done is electrical work.
Therefore, ΔG=−nFEcellΔG=−nFEcell
Considering standard electrode potential, the standard gibbs free energy as follows,
ΔGo=−nFEocellΔGo=−nFEocell
Q. How can the Nernst equation be applied in the calculation of the equilibrium constant of any cell reaction?
Ans: Nernst equation, Ecell=E0cell−RTnFlnQEcell=Ecell0−RTnFlnQ
For any cell reaction in equilibrium, then Q=KcQ=Kc
Where KcKc = equilibrium constant
Ecell=E0cell−RTnFlnKcEcell=Ecell0−RTnFlnKc
Q. The cell reaction as written is spontaneous if the overall EMF of the cell is positive. Comment on this statement.
Ans: If the e.m.f of a cell is positive, then the Gibbs free energy of the overall reaction is less than zero.
ΔG=−nFEcellΔG=−nFEcell
Therefore, if EcellEcell is positive, then the cell reaction is spontaneous.
Q. (a) Following reactions occur at cathode during the electrolysis of aqueous silver chloride solution :
Ag+(aq) + e– → Ag(s) E° = +0.80 V
H+(aq) + e– → 12H2(g) E° = 0.00 V
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why?
(b) Define limiting molar conductivity. Why conductivity of an electrolyte solution decreases with the decrease in concentration?
Answer:
(a) At the cathode Ag+ (aq) + e– → Ag(s)
reaction is feasible, because Ag+ ion has higher reduction potential i.e. higher E° value.
(b) Limiting molar conductivity or the molar conductivity of solution at infinite dilution is the sum of molar conductivity cations and anions.
Conductivity of an electrolyte solution decreases on dilution because number of ions per unit volume decreases.
Q. Calculate the time to deposit 1.27 g of copper at cathode when a current of 2A was passed through the solution of CuSO4.
(Molar mass of Cu = 63.5 g mol-1,1 F = 96500 C mol-1)
Answer:
CuSO4 → Cu+ + SO42-
Cu2+ + 2e– → Cu
63.5 gram of copper is deposited = 2 × 96500 C
1.27 gram of Cu is deposited = 2×9650063.5 × 1.27
= I × t (Q = I × t)
t = 2×96500×1.2763.5×2 = 1930 seconds
Q. The chemistry of corrosion of iron is essentially an electrochemical phenomenon. Explain the reactions occurring during the corrosion of iron in the atmosphere. (Delhi 2011)
Answer:
The mechanism of corrosion is explained on the basis of electrochemical theory. By taking example of rusting of iron, we Refer tothe formation of small electrochemical cells on the surface of iron.
The redox reaction involves
At anode : Fe(S) → Fe2+ (aq) + 2e–
At cathode : H2O + CO2 ⇌ H2CO3 (Carbonic acid)
H2CO3 ⇌2H+ + CO22-
H2O ⇌ H+ + OH–
H+ + e– → H
4H + O2 → 2H2O
Then net resultant Redox reaction is
2Fe(s) + O2 (g) + 4H+ → 2Fe2+ + 2H2O
Q. Determine the values of equilibrium constant (Kc) and ΔG° for the following reaction :
Ni(s) + 2Ag+ (aq) → Ni2+ (aq) + 2Ag(s),
E° = 1.05 V
(1F = 96500 C mol-1) (Delhi 2011)
Answer:
According to the formula
ΔG° = -nFE° = – 2 × 96500 ×1.05
or ΔG° = -202650 J mol-1 = -202.65 KJ mol-1
Now ΔG° ⇒ -202650 J Mol-1
R = 8.314 J/Mol/K, T = 298 K
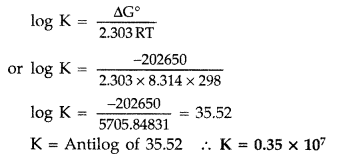
Q. Two half-reactions of an electrochemical cell are given below :
MnO–4 (aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I), E° = 1.51 V
Sn2+ (aq) → Sn4+ (aq) + 2e–, E° = + 0.15 V.
Construct the redox equation from the standard potential of the cell and predict if the reaction is reactant favoured or product favoured. (Delhi 2011)
Answer:
The reactions can be represented at anode and at cathode in the following ways :
At anode (oxidation) :
Sn2+ → = Sn4+ (aq) + 2e– ] × 5 E° = + 0.15 V
At cathode (reduction) :
MnO–4(aq) + 8H+ (aq) + 5e– → Mn2+ (aq) + 4H2O (I)] × 2 E° = + 1.51 V
The Net R × M = 2MnO–4(aq) + 16H+ + 5Sn2+ → 2Mn2+ + 5Sn4+ + 8H2O
Now E°cell = E°cathode – E°anode
= 1.51 – 0.15 = + 1.36 V
∴ Positive value of E°cell favours formation of product.
Q. Explain the term electrolysis. Discuss briefly the electrolysis of
(i). molten NaClNaCl
Ans: Electrolysis: This is a process of decomposition of a chemical compound in aqueous solutions or in a molten state by a chemical change during electric current
The electrolysis of molten NaCl when sodium chloride is melted above 8010C8010C then two electrodes are inserted into the melt and electric current is passed through the molten salt. The following chemical reactions take place at the electrodes.
At cathode: Na++e−→NaNa++e−→Na
At anode: Cl−→12Cl2+e−Cl−→12Cl2+e−
the overall reaction is, 2NaCl→2Na(s)+Cl2(g)2NaCl→2Na(s)+Cl2(g)
(ii). aqueous sodium chloride solution
Ans: Sodium chloride dissolves as Na+Na+ and Cl−Cl− into the water and produces sodium and hydrogen at respective electrodes.
At cathode: H2O(l)+2e−→H2(g)+2OH−H2O(l)+2e−→H2(g)+2OH−
At anode: Cl−→12Cl2+e−Cl−→12Cl2+e−
the overall reaction,
NaCl(aq)+H2O(l)→Na+(aq)+OH−(aq)+H2(g)+12Cl2(g)NaCl(aq)+H2O(l)→Na+(aq)+OH−(aq)+H2(g)+12Cl2(g)
(iii). molten lead bromide
Ans: Molten lead bromide PbBr2PbBr2 is an electrolyte.
At cathode: Pb+2+2e−→Pb(s)Pb+2+2e−→Pb(s)
At anode: 2Br−→Br2+2e−2Br−→Br2+2e−
(iv). water
Ans: The process of water is decomposed into oxygen and hydrogen gas when an electric current is passed through is known as the electrolysis of water.
At cathode: 2H2O(l)+2e−→H2(g)+2OH−;E0=−0.42V2H2O(l)+2e−→H2(g)+2OH−;E0=−0.42V
At anode: 2H2O→O2(g)+4H++4e−;Eo=+0.82V2H2O→O2(g)+4H++4e−;Eo=+0.82V
The overall reaction of electrolysis of water as follows,
2H2O(l)→O2+2H2;Eo=−1.24V2H2O(l)→O2+2H2;Eo=−1.24V
Q. How is electrolytic conductance measured experimentally?
Ans: The resistance between two nodes will give information about the conductance of electrolytes. When electricity passes through electrolyte solution dissociates into positive ions and negative ions. The conductance of electrolytes can be measured by using galvanic cells or the method of electrolysis.
Q. Describe normal hydrogen electrodes and their applications.
Ans: An electrode that is used for reference on all half cell potential reactions is known as a normal hydrogen electrode or standard hydrogen electrode.
The normal hydrogen electrode potential is zero and this is used to calculate the potentials of different half cells and different concentrations.
Q. State and explain Faraday’s laws of electrolysis. What is the electrochemical equivalent?
Ans: First Law of Electrolysis: The mass deposited on any electrode is directly proportional to the quantity of electricity passing through it during electrolysis.
W = zct
Where, W = deposited mass on the electrode
c = current and t = time in sec
If 1 amp current is passed through a solution in one second then the deposited mass of substance on the electrode is equal to its electrochemical equivalent. Here, z is known as the electrochemical equivalent.
Second Law of Electrolysis: The deposited masses on the electrodes is directly proportional to their chemical equivalents when the same quantity of electricity is passed through the different electrolytic cells.
Consider, W1,W2&E1,E2W1,W2&E1,E2be the amount of mass deposited and their chemical equivalents respectively.
According to faraday’s second law of electrolysis,
W1αE1W1αE1 , and W2αE2W2αE2
Then, W1W2=E1E2W1W2=E1E2
Q. What do you understand by ‘electrolytic conduction’? What are the factors on which electrolyte conduction depends? What is the effect of temperature on electrolytic conduction?
Ans: When electric current passes through the electrolytic solutions then the ability of the solutions to allow the current is known as electrolytic conductance.
Factors affecting electrolytic conductance,
- Concentration of ions
- Nature of electrolyte
- Temperature
Effect of temperature: when the temperature changes the electrolyte gets dissolved in solution. Hence, the temperature increases the solubility of electrolytes and increases the electrolytic conduction.
Q. What do you mean by
(i). Negative standard electrode potential
Ans: Negative standard electrode potential means the tendency to get reduce is less than hydrogen or greater ease of oxidation compared to that of hydrogen
(ii). Positive standard electrode potential?
Ans: Positive standard electrode potential means the tendency to get reduce more than hydrogen.
Q. Which cell is generally used in hearing aids? Name the material of the anode, cathode, and electrolyte. Write the reactions involved.
Ans: Mercury cells are used in hearing aids.
At cathode: HgO+H2O+2e−→Hg+2OH−HgO+H2O+2e−→Hg+2OH−
At anode: Zn+OH−→ZnO+H2O+2e−Zn+OH−→ZnO+H2O+2e−
Q. A cell contains two hydrogen electrodes. The negative electrode is in contact with a solution of 10−5H+10−5H+ ions. The emf of the cell is 0.118V0.118V at 298 K298 K . Calculate the concentration of the H+H+ ions at the positive electrode.
Ans:
The cell reaction can be represented as,
Pt|H+(10−5M)1atm∥H+(aM)|1atm|PtPt|H+(10−5M)1atm∥H+(aM)|1atm|Pt
At anode: H2→2H++2e−H2→2H++2e−
Negative polarity, [H+]=10−5M[H+]=10−5M
At cathode: 2H++2e−→H22H++2e−→H2
Ecell=0.05912log[H+][10−5]Ecell=0.05912log[H+][10−5]
0.118=0.0591log[H+][10−5]0.118=0.0591log[H+][10−5]
∴[H+]=10−3M∴[H+]=10−3M
The concentration of the H+H+ ions at the positive electrode = 10−3M10−3M
Q. Crude copper-containing FeFe and AgAg as contaminations were subjected to electrorefining by using a current of 175A for 6.434 min. The mass of anode was found to decrease by 22.260g, while that of the cathode was increased by 22.011g. Estimate the % of copper, iron, and silver in crude copper.
Ans:
22.260−22.011=0.249g22.260−22.011=0.249g of impurity present.
A current of 175A for 6.434 min(386.04sec) corresponds to 175×386.04=67551.75C175×386.04=67551.75C of electricity.
The equivalent of Cu is 31.77g
The mass of Cu that should be deposited = 67551.7596500×31.77=22.239g67551.7596500×31.77=22.239g
Increasing in a mass of anode = 22.011g
22.239−22.011=0.228g22.239−22.011=0.228g corresponds to the mass of Cu that is not deposited. This can be equated to the mass of iron that passed into the solution.
The equivalent mass of Fe =27.75g
The mass of Fe = 0.228×27.75=0.199g0.228×27.75=0.199g
The percentage of Fe = 0.199×10022.26=0.900.199×10022.26=0.90
Q. Iron does not rust even if Zinc coating is broken in a galvanized iron pipe but rusting occurs much faster if tin coating over iron is broken. Explain.
Ans: Fe is less electropositive than Zn. So, as long as Zn is on the surface of the Fe pipe then Zn acts as an anode and the Fe pipe acts as a cathode. As a result of this, rusting of Fe is prevented by Zn.
On the other hand, Zn is more electropositive than Sn, which protects iron until the Sn coating is unbroken. If any pores or breaks are observed, even tin is there the exposed iron gets rusted.
Q. Corrosion is an electrochemical phenomenon, explain.
Ans: When a metal surface is exposed to a wet environment, due to the presence of moisture or air oxidation takes place.
Q. Calculate the pH of following cell: Pt,H2(1atm)|H+(H2SO4)Pt,H2(1atm)|H+(H2SO4), if its electrode potential is 0.03V0.03V .
Ans:
Nernst equation for the given cell,
EH2/H+=E0H2/H+−0.05912log[H+]2PH2EH2/H+=EH2/H+0−0.05912log[H+]2PH2
0.03=0.0−0.0591log[H+]10.03=0.0−0.0591log[H+]1
0.03=0.0591pH0.03=0.0591pH
pH=0.030.0591=0.5076pH=0.030.0591=0.5076
Q. At what pH will hydrogen electrode at 298K298K show an electrode potential of -0.118V, when Hydrogen gas is bubbled at 1atm pressure?
Ans:
From Nernst equation, Ecell=Eocell+0.0591pHEcell=Ecello+0.0591pH
for hydrogen electrode, E0cell=±0.0VEcell0=±0.0V
Then, Ecell=0.0591pHEcell=0.0591pH
Given, Ecell=−0.118VEcell=−0.118V
pH = 0.5008
Q. Electrolysis of the solution of MnSO4MnSO4 in aqueous sulphuric acid is a method for the preparation of MnO2MnO2 as per the chemical reaction
Mn+2(aq)+2H2O→MnO2+ 2H+(aq)+H2 Mn+2(aq)+2H2O→MnO2+ 2H+(aq)+H2
Passing a current of 27 A27 A for 2424 Hrs gives 1 kg1 kg of MnO2MnO2 . What is the current efficiency? What are the reactions occurring at anode and cathode?
Ans: According to faraday’s second law of electrolysis,
weight in grams=equivalent weight×current in amperes×time in seconds96500weight in grams=equivalent weight×current in amperes×time in seconds96500
Molecular weight of MnO2MnO2 = 87g
The oxidation number of Mn in Mn+2Mn+2 =+2
The oxidation number of Mn in MnO2MnO2 =+4
Therefore, the change in the oxidation number = +4-(+2)=+2
Hence, the equivalent weight of MnO2MnO2 = 87g2=43g87g2=43g
Given, weight of MnO2MnO2 = 1Kg =1000g
Current = 27A
Time = 24 hrs = (24×60×60)sec(24×60×60)sec
Substitute the values in the faraday’s second law equation,
1000g=43.5×i×(24×60×60)965001000g=43.5×i×(24×60×60)96500
i =25.67A
The value of current efficiency during the electrolysis of MnSO4MnSO4 can be calculated as,
Current efficiency = 25.6727×100=95.07425.6727×100=95.074
The reaction takes place at cathode,
2H++2e−→H22H++2e−→H2
the reaction takes place at anode,
Mn+2→Mn+4+2e−Mn+2→Mn+4+2e−
Q. What do you mean by Kohlrauch’s law: from the following molar conductivities at infinite dilution λ∞mBa(OH)2=457.6Ω−1cm2mol−1 λm∞ Ba(OH)2=457.6 Ω−1cm2mol−1
λ∞m Ba Cl2= 240.6 Ω−1cm2mol−1 λm∞ Ba Cl2= 240.6 Ω−1cm2mol−1
λ∞m NH4Cl= 129.8 Ω−1cm2mol−1 λm∞ NH4Cl= 129.8 Ω−1cm2mol−1
Calculate λ∞m λm∞ for NH4OHNH4OH
Ans: The Kohlrauch’s law states that,
“An infinite dilution each ion migrates independently of it co-ion and makes its own contribution to the total molar conductivity of an electrolyte irrespective of nature.”
λ∞m(NH4OH)=λ∞NH+4+λ∞OH−λm(NH4OH)∞=λNH4+∞+λOH−∞
=(λ∞NH+4+λ∞Cl−)+12(λ∞Ba2++2λ∞OH−)−12(λ∞Ba2++2λ∞Cl−)=(λNH4+∞+λCl−∞)+12(λBa2+∞+2λOH−∞)−12(λBa2+∞+2λCl−∞)
=λ∞m(NH4Cl)+12(λ∞m(Ba(OH)2))−12(λ∞m(BaCl2))=λm(NH4Cl)∞+12(λm(Ba(OH)2)∞)−12(λm(BaCl2)∞)
∴λ∞m(NH4OH)=129.8+12×457.6−12×240.6∴λm(NH4OH)∞=129.8+12×457.6−12×240.6
= 238.3 Ω−1cm2mol−1 238.3 Ω−1cm2mol−1
Q. Calculate the equilibrium constant for the reaction
Zn + Cd2+↔Zn2++Cd Zn + Cd2+↔Zn2++Cd
If E0Cd+2/Cd=−0.403VECd+2/Cd0=−0.403V
E0Zn+2/Zn=-0.763VEZn+2/Zn0=-0.763V
Antilog 12.1827 Antilog 12.1827
Ans:
E0cell=E0Cd+2/Cd−E0Zn+2/Zn=−0.403V−(−0.763V)=0.360VEcell0=ECd+2/Cd0−EZn+2/Zn0=−0.403V−(−0.763V)=0.360V
n = 2, then the Nernst equation of equilibrium constant and emf of cell
logKc=(nE0cell0.059)=(2×0.3600.059)=12.20logKc=(nEcell00.059)=(2×0.3600.059)=12.20
Kc=antilog(12.20)=1.585×1012Kc=antilog(12.20)=1.585×1012
Q. Predict the products of electrolyzing of the following
A dil. Solution of H2SO4H2SO4 with Pt electrode
Ans: oxygen gas is liberated at anode and hydrogen gas at the cathode.
Reaction at cathode,2H++2e−→H22H++2e−→H2
Reaction at anode, H2O→2H++12O2+2e−H2O→2H++12O2+2e−
Q. An aqueous solution of AgNO3AgNO3 with a silver electrode.
Ans: At the cathode: silver ions will be deposited in preference to hydrogen ions
At anode :Ag→Ag++e−Ag→Ag++e−
Q. Zinc electrode is constituted at 298 K by placing ZnZn rod in 0.1M0.1M aqueous solution of zinc sulphate which is 95 95 dissociated at this concentration. What will be the electrode potential of the electrode given that E0Zn2+/Zn=−0.76VEZn2+/Zn0=−0.76V
Ans: Zn+2+2e−→Zn(s)Zn+2+2e−→Zn(s)
[Zn+2]=0.1×95100=0.095M[Zn+2]=0.1×95100=0.095M
According to Nernst equation,
EZn+2/Zn=E0Zn+2/Zn−0.05912log1[Zn+2]EZn+2/Zn=EZn+2/Zn0−0.05912log1[Zn+2]
=−0.76V−(0.05912log10.095)=−0.76V−(0.05912log10.095)
=−0.76−0.05912×1.0223=−0.7902V=−0.76−0.05912×1.0223=−0.7902V












 CUET UG 2026 Online Registration Started...
CUET UG 2026 Online Registration Started...
 CUET 2026 Free Batches Launched by CUET ...
CUET 2026 Free Batches Launched by CUET ...
 CBSE Date Sheet 2026 for Class 10 & ...
CBSE Date Sheet 2026 for Class 10 & ...






