Table of Contents
The Kerala Board of Public Examinations (KBPE) administered the SSLC Chemistry Paper on March 20, 2024. Question Paper As the exam is over, students look for the SSLC Chemistry Answer Key 2024 so that they can cross-check their response whether it is correct or wrong. Candidates are recommended to bookmark this page, as we provided the Kerala SSLC Chemistry Question Paper 2024 with Answers on this page.
SSLC Chemistry Question Paper 2024
The SSLC Chemistry Question Paper 2024 would follow the syllabus and model paper published by the board. The Kerala Class 10 Chemistry Syllabus includes topics such as the Periodic Table and Electronic Configuration, Gas Laws and Mole Concept, Reactivity Series and Electrochemistry, Metal Production, Non-Metal Compounds, Organic Compound Nomenclature and Isomerism, and Chemical Reactions of Organic Compounds. It is essential that students tackle the previous year’s question papers as well as sample papers to ensure that they understand the idea thoroughly.
Kerala SSLC Chemistry Answer Key 2024: Overview
Our in-house experienced faculty will provide the SSLC Chemistry Answer Key 2024, which is accurate and error-free. The Answer Key includes all of the correct answers as well as the precise question number, allowing students to estimate their score. The Total mark of the Kerala Class 10 Chemistry Paper is 40 marks. The Paper consists of 24 questions which are divided into Five Sections and candidates have 1.5 hours to complete. Let’s take a look at the sslc Chemistry Question Paper 2024 in the table below.
| Particulars | Details |
| Exam Conducting Body | Kerala Pareeksha Bhavan |
| Examination Name | Kerala SSLC Examination 2024 |
| Subject | Chemistry |
| Total Marks of Kerala Class 10 Chemistry Question Paper | 40 Marks |
| Duration | 1.5 Hours |
| Exam timing | from 9:30 to 11: 15 AM |
| Kerala Class 10 Chemistry Exam Date | 15th March 2024 |
Ace your Preparation with CUET 2024 प्रतिज्ञा Science Complete Batch
SSLC Chemistry Answer Key 2024
The Kerala SSLC Chemistry paper 2024 will be held in offline mode on March 19, 2024. After finishing the exam, students frequently look for the Kerala SSLC Chemistry Answer Key 2024 to double-check their answers and compute their grades. After the exam is concluded, our professional faculty will provide you with an error-free and accurate Kerala SSLC Chemistry paper 2024 with Solutions on this website.
SECTION-A
Answer any 4 questions from 1 to 5. Each question carries 1 marks
1. f-block elements which belong to 7th period are known as (Transition elements, Lanthanoids, Halogens, Actinoids)
Answer: Actinoids
2. Write the name of the by product obtained in the industrial production of Soap
Answer: Glycerol
3. The ore of a metal is lighter than the impurities. Which method is suitable for its concentration?
(Levigation, Froth floatation, Magnetic Separation, Leaching)
Answer:Froth floatation
4. When ammonium chloride is heated, a gas with basic nature is obtained. Write the name of the gas
Answer:NH3
5. Which is the product obtained at the cathode when sodium chloride solution is electrolyzed?
(Na, Cl2, O2, H2)
Answer: H2
SECTION-B
Answer any & questions from 6 to 10. Each question carries 2 scores,
Stainless steel, Nichrome and Alnico are alloy steels
6. (a)Which alloy steel is used for the production of heating coils?
Answer: Nichrome
(b) Identify the alloy steels having the same components among these.
Answer: Stainless steel, Nichrome
7. (a) The location state of Mn in MnO2 is 4. Find out the oxidation state of Mn in Mn2O3.
(Hint: Oxidation state of oxygen-2)
Answer: +3
(b) Transition elements show variable oxidation state. Why?
8. The molecular formula of a hydrocarbon is C2H4.
(a) To which category does this hydrocarbon belong? [Alkane, Alkene, Alkyne]
Answer: Alkene
(b) Draw the structure of an alicyclic hydrocarbon with the same molecular formula.
SSLC Chemistry Question Paper Answer Key
Candidates who appeared in the Kerala SSLC 2024 Chemistry Exam, can cross-check their answers with the answer sheet given below.
| Question numbers | Correct Answers |
| 1 | Actinoids |
| 2 | Glycerol |
| 3 | Froth floatation |
| 4 | Ammonia (NH3) |
| 5 | H2 |
| 6 | a.Nichrome. b. Stainless steel & Nichrome |
| 7 | a. +3
b. In d block elements, there is just a minor energy difference between the outermost s subshell and the penultimate d subshell. Under appropriate conditions, electrons in the penultimate d subshell participate in chemical processes alongside the outermost s electrons. |
| 8 | a.Alkene
b. |
| 9. | a.Pressure = 2/2 = 1 atm b. Boyle’s law |
| 10 | a. CH3 – CH2 – COO – CH3 b. Compounds ii & v That is, CH3 – CH2 – COOH & CH3 – OH |
| 11 | a. Contact processes b.Vanadium pentoxide c. Dehydration is a substance’s ability to absorb chemically mixed water or to eliminate hydrogen and oxygen in the same ratio as water. Sulfuric acid is a powerful dehydrating agent. |
| 12 | a. Copper bangle b. Silver nitrate solution. ( Or mixture of silver nitrate and Silver cyanide solution) c. Ag →Ag+ + 1e |
| 13 | a. 6 b. Methyl radical c. 3 – methyl hexane |
| 14 | a. Bauxite b. b. i. Powdered bauxite is added to hot concentrated NaOH solution. Then it is converted to Sodium aluminate solution. ii. After filtering out the contaminants, a tiny amount of Aluminium Hydroxide [Al(OH)3] is added and thoroughly diluted with water. As a result, a significant amount of aluminum hydroxide precipitates. iii. The Al(OH)3 crystals are separated and intensively heated, resulting in their breakdown into Al2O3 (Alumina). 2Al(OH)3 + heat → Al2O3 + 3H2O |
| 15 | a. CH3 – CH3 b. CH3 – CHCl – CH2Cl c. CH2 = CH2 |
| 16 | a.26 b.3d c. Period:4 Group: 8 |
| 17 | a.Ether b.C3H8O c.Methoxy ethane d. CH3 – CH2 – CH2 – OH |
| 18 | a. 22.4 L b. Number of moles = 68/17 = 4 Volume of 68 g Ammonia = 4×22.4 = 89.6 L c. Number of molecules = 4×6.022x |
| 19 | a.When rate of forward reaction and rate of backward reaction becomes equal. b.i. Product decreases. ii. Product increases. Iii. Product increases. |
| 20 | a. Cu & Ag b. Mg & Zn c.i.Zn c.ii.Fe2+ + 2e →Fe |
Kerala SSLC Chemistry Question Paper Last year
Check out the Last year’s SSLC Chemistry Question paper given below.
PART-I
Questions from 1 to 9 carries 1 score each.
A. Answer any four questions from 1 to 6.
1. Identify the compound which contains a carbon-carbon triple bond.
(C5H12, C2H2, C3H6, CH4)
2. Which one of the following subshells has the highest energy?
(1s, 3d, 4s, 3p)
3. Find the relation and fill up suitably.
(a) Tin stone : Magnetic separation
(b) Bauxite :________
4. Which gas is produced when metals react with dilute hydrochloric acid ?
5. 1 GMM of a substance contains _______________ number of molecules
6. What happens to the rates of forward and backward reaction at equilibrium point?
B. Answer all questions from 7 to 9.
7. Which metal is deposited at cathode, when molten sodium chloride is electrolysed? (Sodium, Hydrogen, Chlorine, Calcium)
8. How many electrons are donated by first group elements generally in chemical reactions?
(1, 2, 3, 4)
9. In which electrode aluminium metal is produced during the electrolysis of Alumina?
PART – II
Questions from 10 to 12 carry 2 scores each.
A. Answer the following question.
10. (a) Which are the two compounds formed when Ammonium Chloride (NHCI) is strongly heated?
(b) Write the chemical equation for this reaction.
B. Answer any one question from 11 and 12.
11. Find the mass of 44.8 L of NH3 kept at STP (Hint: Atomic mass N=14, H=1)
12. (a) What is electroplating?
(b) Which is the electrolyte used in electroplating of copper on an iron bangle ?
PART – III
Questions from 13 to 17 carry 3 scores each.
A. Answer any three questions from 13 to 16.
13. (a) Atomic number of an element is 17. Write its subshell electronic configuration.
(b) Find the group number and period number of this element in the periodic table.
14. (a) Molten iron obtained from the blast furnace contains 4% carbon and other impurities. What is this known as ?
(b) Which alloy steel is used for making permanent magnets?
(c) Some alloy steels contain the same component. Then how do they possess different properties?
15. N2(g)+3H2(g) 2NH3(g) + Heat
How do the following changes influence the amount of the product?
(a) Temperature decreases
(b) Pressure increases
(c) Ammonia produced is removed continuously from the system.
16.
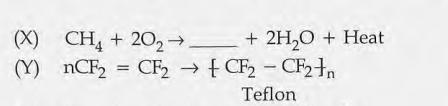 (a) Complete the chemical equation X.
(a) Complete the chemical equation X.
(b) Name the reaction Y.
(c) Write any one use of Teflon.
B. Answer the following questions.
17. (i) CH3-CH2-CH2-CH3
(ii) CH3-CH2-CH2-CH2-OH
(iii) CH3-CH2-O-CH2-CH3
(iv) CH3-CH2-CH3
(a) Identify the isomer pair in the given compounds.
(b) Name the isomerism.
(c) How many isomers are possible for compound (i)?




 CBSE Class 12 Home Science Answer Key 20...
CBSE Class 12 Home Science Answer Key 20...
 CBSE Class 12 History Answer Key 2025, G...
CBSE Class 12 History Answer Key 2025, G...
 CBSE Class 12 Computer Science Answer Ke...
CBSE Class 12 Computer Science Answer Ke...










