Table of Contents
Kolbes Electrolysis
Kolbes electrolysis, also known as Kolbe’s electrolytic synthesis, is a chemical reaction that takes place during the electrolysis of certain carboxylic acids. It was first discovered by Hermann Kolbe in the mid-19th century.
In Kolbes electrolysis, a carboxylic acid is electrolyzed in the presence of an inert electrolyte, such as a concentrated solution of a strong acid. The process involves the oxidation and reduction of carboxylate ions (formed from the carboxylic acid) at the anode and cathode, respectively.
At the anode, carboxylate ions undergo oxidation and lose electrons, resulting in the formation of carbon dioxide gas. The overall reaction at the anode can be represented as:
2RCOO⁻ → R₂ + 2CO₂ + 2e⁻
At the cathode, water molecules are reduced to produce hydrogen gas and hydroxide ions. The overall reaction at the cathode can be represented as:
2H₂O + 2e⁻ → H₂ + 2OH⁻
The net result of Kolbes electrolysis is the decarboxylation of the carboxylic acid, leading to the formation of an alkane with two carbon atoms more than the original carboxylic acid. For example, in the case of acetic acid (CH₃COOH), Kolbe electrolysis would yield ethane (C₂H₆).
Kolbes electrolysis is an important synthetic tool in organic chemistry, allowing for the preparation of higher hydrocarbons from carboxylic acids. It has applications in various areas, including the production of alkanes, alkynes, and alkenes.
Kolbe Reaction for Class 12
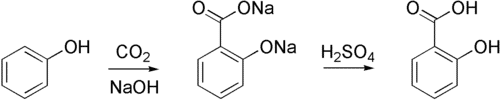
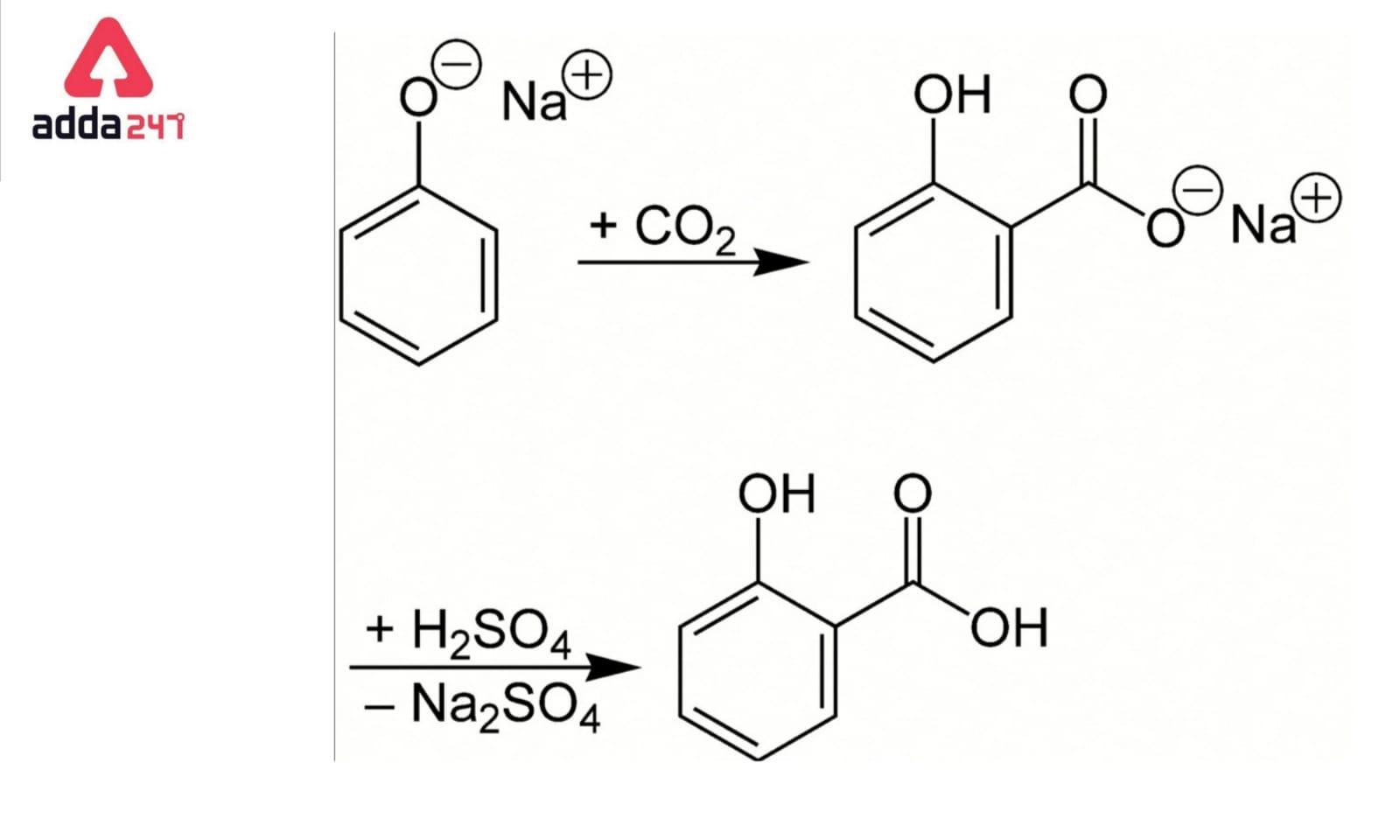
The Kolbe–Schmitt reaction, also known as the Kolbe procedure, is a carboxylation reaction that involves heating sodium phenoxide (the sodium salt of phenol) with carbon dioxide under pressure (100 atm, 125 °C) and then treating the result with sulfuric acid. An aromatic hydroxy acid, commonly known as salicylic acid, is the end product (the precursor to aspirin).
4-hydroxybenzoic acid, a key precursor for the diverse paraben class of biocides used in personal care products, can be produced by employing potassium hydroxide.
The process is also employed in the industrial synthesis of 3-hydroxy-2-naphthoic acid; the carboxylation regiochemistry is temperature sensitive in this case.
Kolbe Reaction Definition
Kolbe reaction, often called Kolbe Schmitt reaction, is an addition reaction named after Hermann Kolbe and Rudolf Schmitt. The phenoxide ion is formed when phenol is treated with sodium hydroxide. When it comes to electrophilic aromatic substitution reactions, the phenoxide ion produced is more reactive than phenol. As a result, it conducts an electrophilic substitution reaction with the weak electrophile carbon dioxide. The principal result is ortho-hydroxybenzoic acid (salicylic acid). Kolbe’s reaction is the common name for this reaction.
Kolbe Reaction Mechanism
The salicylate is produced by the nucleophilic addition of a phenoxide, often sodium phenoxide (NaOC6H5), to carbon dioxide in the Kolbe–Schmitt reaction. The reaction (protonation) of the salicylate anion with an acid to create the required salicylic acids is the final step (ortho- and para- isomers).
Kolbes Reaction Applications for Class 12
The applications of Kolbes Reaction are as follows:
- 4-Hydroxybenzoic acid can be obtained when potassium hydroxide is employed in the Kolbe reaction. This is a crucial step in the production of parabens (parahydroxybenzoate or ester of para-hydroxy benzoic acid, used as a biocide in cosmetic products).
- The Kolbe reaction can also be used to make 3-hydroxy-2-naphthoic acid, which is a common precursor of azo dyes and pigments in the industry.
- By coupling salicylic acid with acetic anhydride, aspirin can be produced. Aspirin is a popular pain reliever.
What is kolbe Synthesis?
Kolbe synthesis, also known as Kolbe-Schmitt reaction, is a chemical reaction that involves the electrolysis of a phenol or phenolate salt in the presence of carbon dioxide (CO2). It is named after Hermann Kolbe and Rudolf Schmitt, who contributed to its development.
The Kolbe synthesis proceeds as follows:
- Electrolysis: A phenol (or phenolate salt) is subjected to electrolysis in an aqueous or alcoholic solution. An electric current is passed through the solution using suitable electrodes.
- Electrophilic Substitution: At the anode, the phenol or phenolate undergoes electrophilic substitution. The hydroxyl group (-OH) of the phenol is oxidized, and the resulting phenoxyl radical (PhO•) reacts with carbon dioxide (CO2) to form a salicylate intermediate.
- Rearrangement: The salicylate intermediate then undergoes rearrangement through decarboxylation and intramolecular proton transfer, leading to the formation of a new aromatic ring.
- Acidification: The reaction mixture is then acidified to protonate the hydroxyl group of the intermediate, resulting in the formation of the final product, which is a salicylic acid.
The Kolbe synthesis is an important method for the preparation of salicylic acids, which find applications in various industries, including pharmaceuticals, cosmetics, and as intermediates in organic synthesis.
It’s worth noting that the term “Kolbe synthesis” can also refer to other reactions or processes developed by Hermann Kolbe, as he made significant contributions to organic chemistry.
Kolbe’s Reaction Class 12 in Hindi
कोल्बे-श्मिट प्रतिक्रिया, जिसे कोल्बे प्रक्रिया के रूप में भी जाना जाता है, एक कार्बोक्सिलेशन प्रतिक्रिया है जिसमें दबाव में कार्बन डाइऑक्साइड के साथ सोडियम फिनॉक्साइड (फिनोल का सोडियम नमक) को गर्म करना (100 एटीएम, 125 डिग्री सेल्सियस) और फिर सल्फ्यूरिक एसिड के साथ परिणाम का इलाज करना शामिल है। . एक सुगंधित हाइड्रॉक्सी एसिड, जिसे आमतौर पर सैलिसिलिक एसिड के रूप में जाना जाता है, अंतिम उत्पाद (एस्पिरिन का अग्रदूत) है।
4-हाइड्रॉक्सीबेन्जोइक एसिड, व्यक्तिगत देखभाल उत्पादों में उपयोग किए जाने वाले बायोसाइड्स के विविध पैराबेन वर्ग के लिए एक प्रमुख अग्रदूत, पोटेशियम हाइड्रॉक्साइड को नियोजित करके उत्पादित किया जा सकता है।
प्रक्रिया 3-हाइड्रॉक्सी-2-नैफ्थोइक एसिड के औद्योगिक संश्लेषण में भी कार्यरत है; इस मामले में कार्बोक्सिलेशन रेजियोकेमिस्ट्री तापमान संवेदनशील है।
कोल्बे प्रतिक्रिया: परिभाषा
कोल्बे प्रतिक्रिया, जिसे अक्सर कोल्बे श्मिट प्रतिक्रिया कहा जाता है, हरमन कोल्बे और रुडोल्फ श्मिट के नाम पर एक अतिरिक्त प्रतिक्रिया है। जब फिनोल की सोडियम हाइड्रॉक्साइड के साथ क्रिया की जाती है तो फीनॉक्साइड आयन बनता है। जब इलेक्ट्रोफिलिक सुगंधित प्रतिस्थापन प्रतिक्रियाओं की बात आती है, तो उत्पादित फिनॉक्साइड आयन फिनोल की तुलना में अधिक प्रतिक्रियाशील होता है। नतीजतन, यह कमजोर इलेक्ट्रोफाइल कार्बन डाइऑक्साइड के साथ एक इलेक्ट्रोफिलिक प्रतिस्थापन प्रतिक्रिया करता है। मुख्य परिणाम ऑर्थो-हाइड्रॉक्सीबेन्जोइक एसिड (सैलिसिलिक एसिड) है। इस प्रतिक्रिया का सामान्य नाम कोल्बे की प्रतिक्रिया है।
कोल्बे प्रतिक्रिया: तंत्र
सैलिसिलेट कोल्बे-श्मिट प्रतिक्रिया में कार्बन डाइऑक्साइड के लिए एक फिनॉक्साइड, अक्सर सोडियम फेनोक्साइड (NaOC6H5) के न्यूक्लियोफिलिक जोड़ द्वारा निर्मित होता है। आवश्यक सैलिसिलिक एसिड बनाने के लिए एक एसिड के साथ सैलिसिलेट आयन की प्रतिक्रिया (प्रोटॉनेशन) अंतिम चरण (ऑर्थो- और पैरा-आइसोमर) है।
कोल्बे प्रतिक्रिया: अनुप्रयोग
कोल्बे रिएक्शन के अनुप्रयोग इस प्रकार हैं:
4-हाइड्रॉक्सीबेन्जोइक अम्ल तब प्राप्त किया जा सकता है जब कोल्बे अभिक्रिया में पोटैशियम हाइड्रॉक्साइड का प्रयोग किया जाता है। यह पैराबेंस (पैराहाइड्रॉक्सीबेन्जोएट या पैरा-हाइड्रॉक्सी बेंजोइक एसिड का एस्टर, कॉस्मेटिक उत्पादों में बायोसाइड के रूप में उपयोग किया जाता है) के उत्पादन में एक महत्वपूर्ण कदम है।
कोल्बे प्रतिक्रिया का उपयोग 3-हाइड्रॉक्सी-2-नैफ्थोइक एसिड बनाने के लिए भी किया जा सकता है, जो उद्योग में एज़ो डाई और पिगमेंट का एक सामान्य अग्रदूत है।
सैलिसिलिक एसिड को एसिटिक एनहाइड्राइड के साथ जोड़कर एस्पिरिन का उत्पादन किया जा सकता है। एस्पिरिन एक लोकप्रिय दर्द निवारक है।
Related Post:
- PCS Full Form- Provincial Civil Service
- Laws Of Motion By Newton- First, Second, Third, Applications
- Full Wave Rectifier- Formula, Working, Efficiency, Circuit
- Decomposition Reaction- Formula, Example For Class 10
- Heron’s Formula For Area Of Triangle, Proof And Example




 VITEEE Admit Card 2025 Out Today, Downlo...
VITEEE Admit Card 2025 Out Today, Downlo...
 NEET Organic Chemistry Syllabus 2025, Ch...
NEET Organic Chemistry Syllabus 2025, Ch...
 NTA NEET Exam Date 2025 OUT, Exam Timing...
NTA NEET Exam Date 2025 OUT, Exam Timing...










