Table of Contents
Lava Lamp: Have you ever seen the fascinating lava lamp? In a lava lamp, enormous colored bubbles rise, drop, and transform. What if you could construct this stunning aesthetically pleasing lava lamp yourself for less money? On the other hand, you may create this gorgeous lava lamp experiment for science projects for class 6, 10 and astonish your friends and teachers. Yes, you can create a lava lamp for your science exhibition project or for home decorating utilizing items you already have at home. This is a wonderful science project ideas for class 10, 6 that illustrates the principles of liquid density and chemical mechanisms. If you’re ready, let’s get started on creating this lava lamp right away.
Lava Lamp Experiment
Lava lamps are frequently referred to as liquid motion lighting. The density and solubility of two liquids are discussed in the lava lamp science model for class 10 hypothesis. This lava lamp experiment explanation helps figure out why oil and water do not mix.
The liquids in the science project of the lava lamp experiment are insoluble in one another and have densities that are quite close to one another. Because oil and water are insoluble in each other, many people utilize them together. However, the densities of the two liquids are quite different. To make the lava lamp science project work, you must find two liquids with relatively close densities. carry out the lava lamp experiment to clearly understand the functioning concept of the science model for class 10.
Science Projects for Class 6, Class 10 – Lava Lamp Experiment
A lava lamp usually begins to work an hour after being turned on. When we have located the two liquids (water and oil) in the lava lamp experiment, we will heat the bottom of the combination with a light bulb. The heat will be absorbed and expanded by the denser liquid. It becomes less dense as it expands. Because the densities of the liquids are identical, the earlier denser liquid becomes lighter and rises above the other liquid. All of this happens in a steady state, and the density variations are very minor. This science project for class 6, class 10 technique will be covered in full in the next part.
Lava Lamp Experiment
As previously stated, the lava lamp experiment operates on the density and polarity principles. Let us take a closer look at the materials required for the lava lamp experiment as well as the step-by-step procedure for successfully completing the science project for class 6, and class 10.

Lava Lamp Experiment Ingredients
- Water
- a clean plastic container( preferably one with a smooth exterior)
- Food coloring
- Vegetable oil (alternatively, mineral or baby oil)
- Fizzing tablets (such as Alka Seltzer)
Procedure to make Lava Lamp
Step 1: At first fill about a quarter of the bottle with water.
Step 2: Fill the bottle close to the top with vegetable oil. You might use a funnel or a measurement vessel with a spout. It can take a few minutes for the oil and water to separate out.
Step 3: Mix in a few drops of your preferred food coloring. Keep an eye on how the color penetrates through the oil. Now notice that the color drops immediately mix with the water or float for a few minutes.
Step 4: Cut your fizzy tablet into little pieces and place some of them in the bottle. The bubbles will now begin to blob.
Step 5: students can even acquire a torch, turn off the lights, and place another half-tablet inside.
Step 6: It’s time to flash the torch over the lava lamp when the lumps are boiling; the lava lamp will be spectacular to watch now.
Observation
What we’ll observe now is a little bubble rising to the surface, carrying part of the water with it. The exciting part now is when the air bubbles on top dissolve and the color returns. In the following section, we will discover the reason behind this lava lamp science project.
Lava Lamp Experiment Explanation
Lava Lamp Experiment Explanation:
- Due to its lighter or less dense composition compared to water, the oil stays afloat on top of the water. Because food coloring has the same density as water, it sinks through the oil and combines with it.
- As you drop the tablet in, it settles to the bottom and begins to dissolve. As it dissolves, it emits carbon dioxide gas. Because gas or air is lighter than water, it hovers to the surface. The air bubbles carry some colored water to the surface.
- When the air escapes from the colored water blob, the water becomes heavy and sinks. It repeats this process until the tablet is entirely dissolved.
- Add more fizzing tablets to run this Lava lamp experiment for a longer period of time.
Lava Lamp Experiment Aims for kids
Real lava lamps, like our lava lamp, use polar and non-polar liquids. This lava lamp operates on two distinct scientific principles: density and polarity. Let us now learn these two principles.
Learning 1: What is Density?
The density of a substance is an assessment of how compact it is or the quantity of it fits in a given amount of space.
Formula: density = mass/volume
- We utilized oil and water in our lava lamp experiment. If you weigh an equal volume of oil and water, you’re going to discover that the water is heavier than the identical amount of oil.
- This is due to the fact that water molecules are more closely packed; a cup of water actually possesses more mass than a cup of oil.
- Because water is denser than oil, it can sink to the bottom of a container containing both. Temperature has an effect on density; the more heated a liquid is, the less dense it is.
Learning 1: What is Polarity?
The property of having two oppositely charged sides is known as polarity.
- Water molecules are referred to as “polar” because they carry an asymmetric electrical charge that pulls other atoms. The molecule’s end containing the two hydrogen atoms is charged positively. The opposite end, which contains the oxygen, is a negative charge.
- The positive end of the water molecule will interact with the negative end of other molecules in the same way that north poles are attracted to south poles in a magnet.
- Oil molecules, on the other hand, are non-polar—they have neither positive nor negative charge—and hence are not attracted to water molecules at all.
- Because of this, oil and water don’t mix and in the lava lamp experience we also observed that oil and water remain separate.

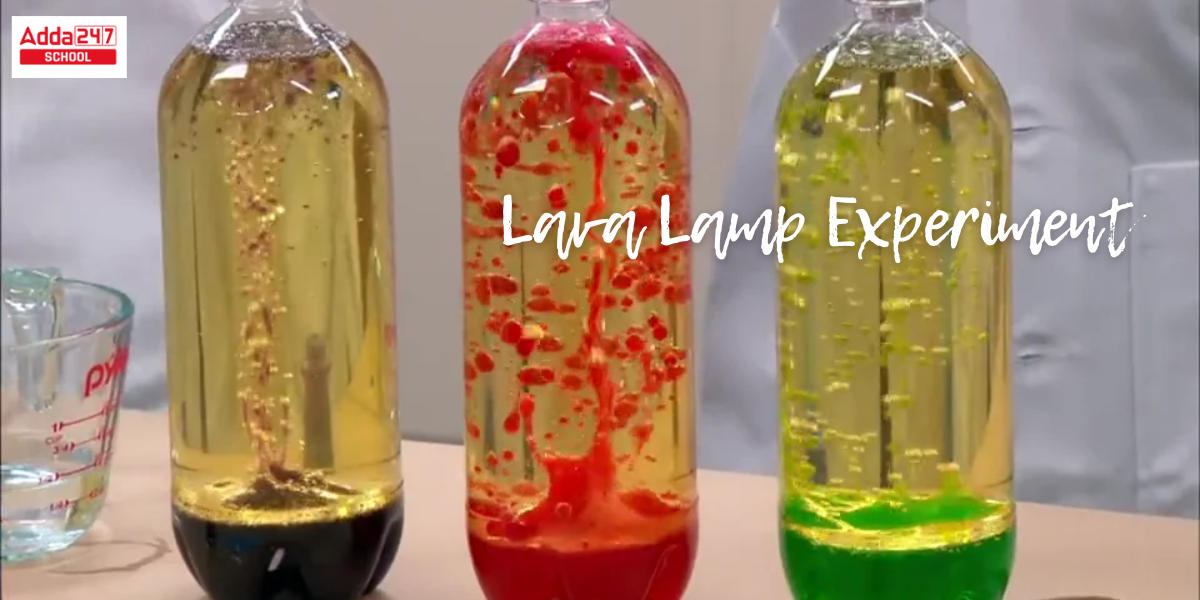

 NEET UG 2025: Is NEET Previous years Que...
NEET UG 2025: Is NEET Previous years Que...
 JEE Mains Session 2 Result 2025 OUT, Sco...
JEE Mains Session 2 Result 2025 OUT, Sco...
 TS Inter Results 2025 Date for TSBIE 1st...
TS Inter Results 2025 Date for TSBIE 1st...










