Table of Contents
Nasal Vaccine- Govt approves Bharat Biotech’s Intranasal Vaccine
Nasal Vaccine: The Union Health Ministry has approved the intra- Nasal vaccine for Covid from Bharat Biotech as a booster dose for people over the age of 18, according to official sources on Friday.
As per the official Statement, Private clinics will offer the needle-free shot of the Nasal vaccine, and from Friday night, it will be made available on the Co-WIN platform.
Check out: Biomedical Waste Management Project Explanation
Nasal Vaccine Covid
Nasal Vaccine Covid India: Many countries are developing vaccines that are typically injected intravenously or intramuscularly to stop the pandemic from spreading throughout the world. However, some organizations, like Biotech and Serum right now, are also employing a different strategy – developing to target the virus faster.
After almost 2 years of the Covid wave, again a new variant of Covid is responsible for the spike in Covid cases in China in recent times.
While millions of deaths are expected in China in the coming months, the situation there is concerning, experts are optimistic that India’s situation will be different.
The Center and the states have increased their precautions against the contagious variant in response to the discovery of four cases of BF.7 in India.
Also Check: Sustainable Development Project, Definition, Example
Nasal Vaccine Name
The nasal vaccine developed by Bharat Biotech is known as iNCOVACC (BBV 154). Incovacc is a “recombinant replication-deficient adenovirus vectored vaccine with a pre-fusion stabilized spike protein” developed by Bharat Biotech.
According to the manufacturer, it stimulates a broad immune response by neutralising IgG, mucosal IgA, and T cell responses. It also stimulates immune responses at the site of infection (in the nasal mucosa), which is critical for preventing Covid-19 infection and transmission.
This vaccine has been specifically designed for intranasal administration via nasal drops. The nasal delivery system was created to be cost-effective in low and middle-income countries.
Nasal Vaccine Covid India
As, India had administered more than 2.19 billion doses of all currently approved vaccines as of 2 December 2022, including the first, second, and booster doses. In India, 88% of the eligible population (12+) is fully immunized and 95% of the eligible population (12+) has received at least one vaccination.
And Now the Union health ministry has authorized Bharat Biotech’s nasal vaccine on Friday,24 December 2022.
When will Nasal Vaccine be available?
As the number of Covid-19 cases grows in many parts of the world, the health ministry has authorized Bharat Biotech’s nasal vaccine against the viral infection, stating on Friday that the needle-free vaccine will be available at private clinics and will be made available on the Co-WIN platform on Friday evening.
Nasal Vaccine Manufacturers in India- Covid Updates
According to Bharat Biotech, iNCOVACC (BBV 154) has received approval from the Central Drugs Standard Control Organization (CDSCO) in India for heterologous booster doses under Restricted Use in Emergency Situations for ages 18 and above.
In September, Bharat Biotech signed a licensing agreement with Washington University School of Medicine (WUSM) for a novel chimp-adenovirus, single-dose intranasal Covid vaccine. The intranasal vaccine had previously received approval under Restricted Use in Emergency Situation for ages 18 and up for the primary 2-dose schedule. At 14 trial sites across India, 3100 subjects were tested for safety and immunogenicity in phase III trials.
The Union Health Ministry has approved Bharat Biotech’s intranasal Covid vaccine as a booster dose for those over the age of 18, according to official sources.
Who will be Eligible for Nasal Vaccine?
The nasal vaccine can only be used by those who have already received the two doses of the vaccine. The government has made it clear that the current approval is for this vaccine to be used only as a precautionary dose in those over the age of 18.
You cannot use this if you have already taken a precautionary dose. This precautionary dose is only available to those who have received two doses of Covishield, Covaxin, or, in some cases, the Sputnik V.
Only about 30% of those who received both doses received the precautionary dose, and as it appeared that Covid had declined, people were hesitant. So those are the people who are eligible.
Nasal Vaccine Approval
The Drugs Controller General of India approved the nasal vaccine, BBV154, in November for limited use as a heterologous booster dose in an emergency situation for adults. The vaccine’s approval coincides with an increase in Covid cases in China and some other nations.
According to official sources on Friday, the Covid Nasal Vaccine from Bharat Biotech has been authorized by the Union Health Ministry as a booster dose for people over the age of 18.
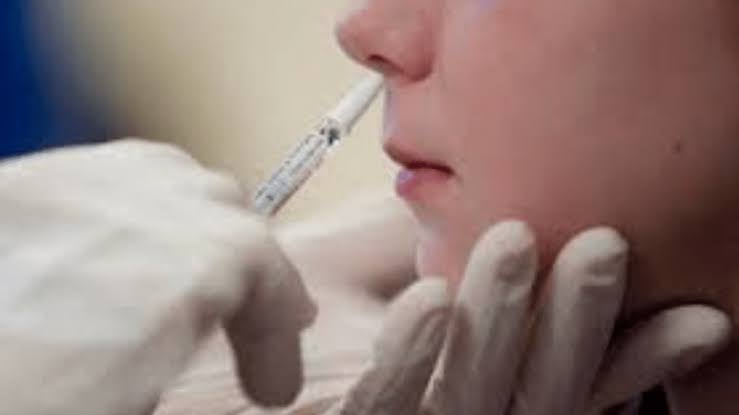
Nasal Covid Vaccine: How it Works?
Nasal Vaccine: The virus normally enters your body through the nose, the nasal vaccine causes you to produce proteins in your blood and nose that help you fight the virus.
- A doctor uses a tiny, needle-free syringe to spray the vaccine into your nostrils. Normally, it takes two weeks for it to begin to work.
- Some claim that it makes sense to develop vaccines for the airway in addition to the more common shots given the severity and quick spread of the.
Advantages of Nasal Vaccine
Take a look at the advantages of intra-nasal vaccines and Bharat Biotech’s Incovacc:
- A broad immune response, including neutralizing IgG, mucosal IgA, and T-cell responses, is elicited by an intranasal vaccine.
- The procedure is non-invasive and needle-free.
- As the nasal mucosa has a well-organized immune system, the nasal route has excellent potential for vaccination.
- Immune reactions at the injection site (in the nasal mucosa) are crucial for preventing COVID-19 infection and transmission.
- High compliance (suitable for both adults and children).
- As a result, it eliminates needle-related risks such as injuries and infections.
- It does not require trained healthcare workers due to its simple delivery mechanism.
- Manufacturing that is scalable and can satisfy global demand


 CUET PG Result 2025 Soon @exams.nta.ac.i...
CUET PG Result 2025 Soon @exams.nta.ac.i...
 NEET Admit Card 2025 Release Date, How t...
NEET Admit Card 2025 Release Date, How t...
 CUET UG 2025 Vs CUET UG 2024, Check Majo...
CUET UG 2025 Vs CUET UG 2024, Check Majo...










