Table of Contents
The NCERT Solutions for Class 12 Chemistry Chapter 6 consists of solutions to various important questions. Students are strongly advised to study each topic carefully to develop a complete grasp of the concepts covered in the chapter and effectively utilize the offered solutions. Students should also revise Class 12 Chemistry NCERT Solutions given on this page.
Candidates can grasp the principles of IUPAC naming for Haloalkanes and Haloarenes. They will also study the uses of organometallic reactions, the synthesis of haloalkanes and haloarenes, as well as stereochemistry. The students additionally learn how to design the reaction mechanism.
Class 12 Chemistry Chapter 6 NCERT Solutions
Haloalkanes and Haloarenes deal with the study of important methods of preparation, physical and chemical properties, and uses of organohalogen compounds. After studying this chapter students will be able to name haloalkanes and haloatrenes according to the IUPAC system of nomenclature from their given structures.
Describe the reactions involved in the preparation of haloalkanes and haloarenes with various types of reactions; use stereochemistry as a tool for understanding the reaction mechanism; appreciate the applications of organometallic compounds and highlight the environmental effect of polyhalogen compounds.
Haloalkanes are hydrocarbons containing aliphatic alkane with one or more hydrogen atom/s replaced by halogens. Haloarenes are hydrocarbons containing aromatic alkane with one or more hydrogen atom/s replaced by halogens. These are open-chain hydrocarbon compounds. These are closed chain hydrocarbon compounds.
Uses of Haloalkanes and Haoarenes
Haloalkanes and haloarenes are used for many industrial and day-to-day purposes. They are used as flame retardants, propellants, solvents, pharmaceuticals, refrigerants, fire extinguishants, and many more. They are used as solvents for non-polar compounds.
Haloalkanes and haloarenes are chemical compounds derived from alkanes and arenes containing more halogens. The NCERT class 12 solutions of this chapter broadly discuss the definitions, classifications, examples and applications, and other subtopics under Haloalkanes and Haloarenes.
NCERT Solutions Class 12 Chemistry Chapter 6
Q) Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl, vinyl, or aryl halides:
- (CH3)2CHCH(Cl)CH3
- CH3CH2CH(CH3)CH(C2H5)Cl
- CH3CH2C(CH3)2CH2I
- (CH3)3CCH2CH(Br)C6H5
- CH3CH(CH3)CH(Br)CH3
- CH3C(C2H5)2CH2Br
- CH3C(Cl)(C2H5)CH2CH3
- CH3CH=C(Cl)CH2CH(CH3)2
- CH3CH=CHC(Br)(CH3)2
- P-ClC6H4CH2CH(CH3)2
- M-ClCH2C6H4CH2C(CH3)3
- O-Br-C6H4CH(CH3)CH2CH3
Answer: The IUPAC names of various compounds are given below in order:
(i) 2-Chloro-3-methyl butane (secondary alkyl halide)
(ii) 3-Chloro-4-methyl hexane (secondary alkyl halide)
(iii) 1-Iodo-2,2-dimethylbutane (primary alkyl halide)
(iv) 1-Bromo-3,3-dimethyl-1-phenylbutane (secondary benzyl halide)
(v) 2-Bromo-3-methylbutane (secondary alkyl halide)
(vi) 1-Bromo-2-ethyl-2-methylbutane (primary alkyl halide)
(vii) 3-Chloro-3-methylpentane (tertiary alkyl halide)
(viii) 3-Chloro-5-methylhex-2-ene (vinyl halide)
(ix) 4-Bromo-4-methylpent-2-ene (allyl halide)
(x) 1-Chloro-4-(2-methylpropyl) benzene (aryl halide)
(xi) 1-Chloromethyl-3-(2,2-dimethylpropyl) benzene (primary benzyl halide)
(xii) 1-Bromo-2-(1-methylpropyl) benzene (aryl halide)
Q) Give the IUPAC names of the following compounds :
- CH3CH(Cl)CH(Br)CH3
- CHF2CBrClF
- ClCH2C=CCH2Br
- (CCl3)3CCl
- CH3C(p-ClC6H4)2CH(Br)CH3
- (CH3)3CCH=ClC6H4I-p
Answer: The IUPAC names of various compounds are given below in order:
(i) 2-Bromo-3-chlorobutane
(ii) 1-Bromo-1-chloro-1,22-trifluoroethane
(iii) 1-Bromo-4-chlorobut-2-yne
(iv) 2-(Trichloromethyl)-1,1,1,2,3,3,3-heptachloropropane
(v) 2-Bromo-3,3-bis (4-chlororphenyl) butane
(vi) 1-Chloro-1-(4-iodophenyl)-3,3-dimethylbut-1-ene
Q) Which one of the following has the highest dipole moment?
- CH2Cl2
- CHCl3
- CCl4
Answer: Dichloromethane has highest dipole moment among CH2Cl2, CHCl3 and CCl4. The decreasing order of dipole moments is CH2Cl2 > CHCl3 > CCl4. These molecules have tetrahedral geometry due to sp3 hybridization of carbon atom.In CCl4, the individual C−Cl bond dipoles cancel each other which results in zero dipole moment.
Hence, CCl4 is non polar.
Q) What are ambient nucleophiles? Explain with an example.
Answer: The nucleophiles that can attack through two different sites are known as ambident nucleophiles. For example, cyanide ion is an ambident nucleophile. It can attack through either C atom or N atom to form alkyl cyanide or alkyl isocyanide.
Q) Which compound in each of the following pairs will react faster in SN2 reaction with -OH?
- CH3Br or CH3I
- (CH3)3CCl or CH3Cl
Answer:
i.) In SN2 mechanism, the order in which the halides react to some alkyl group is constant. This is because the halide ion becomes a better leaving group when the size of the ion increases.
R-F << R-Cl<< R-Br<< R-I
Hence, the reactivity of CH3I is faster as compared to CH3Br in SN2 reaction with OH-.
ii.) In SN2 mechanism, the nucleophile attacks at the atom bearing the leaving group. The attack of nucleophile in (CH3)3Cl at the carbon atom is hindered as the carbon atoms contain bulky group.
However, CH3Cl does not consist of bulky substituents on the carbon atom bearing the leaving group.
Hence, the reactivity of CH3Cl is faster as compared to (CH3)3Cl in SN2 reaction with OH-.
Q) Explain why
- The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
- Alkyl halides ,though polar, are immiscible with water?
- Grignard reagents should be prepared under anhydrous conditions?
Answer:
i.)In chlorobenzene, the Cl-atom is linked to a sp2 hybridized carbon atom. In cyclohexyl chloride, the Cl-atom is linked to a sp3 hybridized carbon atom. Now, sp2 hybridized carbon has more s-character than sp3 hybridized carbon atom. Therefore, the former is more electronegative than the latter. Therefore, the density of electrons of C – Cl bond near the Cl-atom is less in chlorobenzene than in cydohexyl chloride.
Moreover, the – R effect of the benzene ring of chlorobenzene decreases the electron density of the C – Cl bond near the Cl-atom. As a result, the polarity of the C – Cl bond in chlorobenzene decreases. Hence, the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
(ii) To be miscible with water, the solute-water force of attraction must be stronger than the solute-solute and water-water forces of attraction. Alkyl halides are polar molecules and so held together by dipole-dipole interactions. Similarly, strong H-bonds exist between the water molecules. The new force of attraction between the alkyl halides and water molecules is weaker than the alkyl halide-alkyl halide and water-water forces of attraction. Hence, alkyl halides (though polar) are immiscible with water.
(iii) Grignard reagents are very reactive. In the presence of moisture, they react to give alkanes.
Therefore, Grignard reagents should be prepared under anhydrous conditions.
Q) Give the uses of freon 12, DDT, carbon tetrachloride and iodoform .
Answer: Uses of Freon – 12
Freon-12 (dichlorodifluoromethane, CF2Cl2) is commonly known as CFC. It is used as a refrigerant in refrigerators and air conditioners. It is also used in aerosol spray propellants such as body sprays, hair sprays, etc. However, it damages the ozone layer. Hence, its manufacture was banned in the United States and many other countries in 1994.
Uses of DDT
DDT (p, p-dichlorodiphenyltrichloroethane) is one of the best known insecticides. It is very effective against mosquitoes and lice. But due its harmful effects, it was banned in the United States in 1973.
Uses of carbon tetrachloride (CCl4)
(i) It is used for manufacturing refrigerants and propellants for aerosol cans.
(ii) It is used as feedstock in the synthesis of chlorofluorocarbons and other chemicals.
(iii) It is used as a solvent in the manufacture of pharmaceutical products.
Uses of iodoform (CHI3)
Iodoform was used earlier as an antiseptic, but now it has been replaced by other formulations-containing iodine-due to its objectionable smell. The antiseptic property of iodoform is only due to the liberation of free iodine when it comes in contact with the skin.
Q) Arrange the compounds of each set in order of reactivity towards SN2 displacement :
- 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
- 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 3-Bromo-2-methylbutane III. 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane.
Answer: An SN2 reaction involves the approaching of the nucleophile to the carbon atom to which the leaving group is attached. When the nucleophile is sterically hindered, then the reactivity towards SN2 displacement decreases. Due to the presence of substituents, hindrance to the approaching nucleophile increases in the following order.
1-Bromopentane < 2-bromopentane < 2-Bromo-2-methylbutane
Hence, the increasing order of reactivity towards SN2 displacement is:
2-Bromo-2-methylbutane < 2-Bromopentane < 1-Bromopentane
(ii) Since steric hindrance in alkyl halides increases in the order of 1° < 2° < 3°, the increasing order of reactivity towards SN2 displacement is 3° < 2° < 1°.
Hence, the given set of compounds can be arranged in the increasing order of their reactivity towards SN2 displacement as:
2-Bromo-2-methylbutane < 2-Bromo-3-methylbutane < 1-Bromo-3-methylbutane
(iii) The steric hindrance to the nucleophile in the SN2 mechanism increases with a decrease in the distance of the substituents from the atom containing the leaving group. Further, the steric hindrance increases with an increase in the number of substituents. Therefore, the increasing order of steric hindrances in the given compounds is as below:
1-Bromobutane < 1-Bromo-3-methylbutane < 1-Bromo-2-methylbutane < 1-Bromo-2, 2-dimethylpropane
Hence, the increasing order of reactivity of the given compounds towards SN2 displacement is:
1-Bromo-2, 2-dimethylpropane < 1-Bromo-2-methylbutane < 1-Bromo-3- methylbutane < 1-Bromobutane
Q) p-Dichlorobenzene has higher m.p. and solubility than those of o-and m- isomers. Discuss.
Answer: In the case of dichlorobenzenes, the para isomer is more symmetrical than ortho and meta isomers. Hence, in the crystal lattice, para isomer fits more closely than ortho and meta isomers.
Due to this, more energy is required to break the crystal lattice of the para isomer. Hence, p-Dichlorobenzene has a higher melting point than those of o- and m-isomers.
Q) The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohol but in the presence of alcoholic KOH, alkenes are major products. Explain.
Answer: R−Cl + KOH(aq) à R−OH + KCl
The ionization of aqueous KOH produces hydroxide ions which are strong nucleophiles. Hence, alkyl chlorides undergo substitution to form alcohol.
R−CH2−CH2−Cl + KOH(alc) à R−CH=CH2 + KCl + H2O
Alcoholic KOH solution gives alkoxide ion which is a strong base. It abstracts β hydrogen atom of alkyl chloride. A molecule of HCl is eliminated and an alkene is formed.
The basicity of hydroxide ion is much lower than the basicity of alkoxide ion as hydroxide ion is significantly hydrated in aqueous solution.
Hence, hydroxide ion cannot abstract β hydrogen atom of alkyl chloride.
Q) What are ambident nucleophiles ? Explain with an example.
Ans: Nucleophiles which can attack through two different sites are called ambident nucleophiles. For example, cyanide ion is a resonance hybrid of the following two structures:
![]()
It can attack through carbon to form cyanide and through N to form is O cyanide.
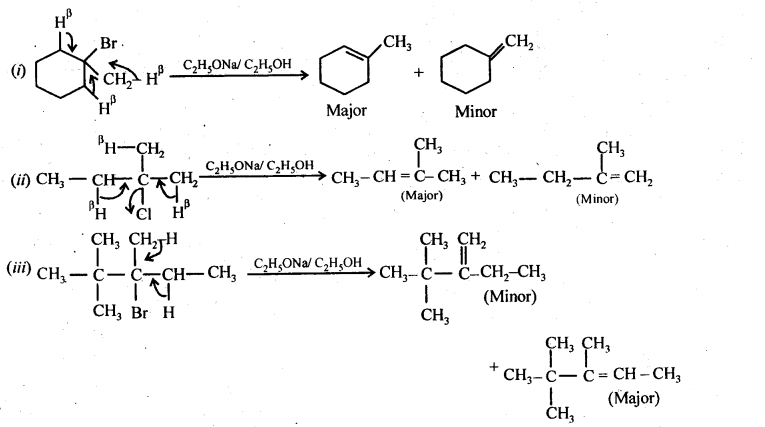
Q) Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(I) 1-Bromo-l-methylcyclohexane
(ii) 2-Chloro-2-methylbutane.
(iii) 2,2,3-Trimethyl-3-bromopentane.
Ans:
Q) Which compound in each of the following-pairs . will react faster in SN2 reaction with -OH? (i)CH3Br or CH3I
(ii)(CH3)3CCl or CH3Cl
Ans: (i)Since I– ion is a better leaving group than Br- ion, therefore, CH3I reacts faster CH3Br in SN2 reaction with OH– ion.
(ii)On steric grounds, 1° alkyl halides are more reactive than tert-alkyl halides in SN2 reactions. Therefore, CH3CI will react at a faster rate than (CH3)3CCl in a SN2 reaction with OH– ion.
Key features of NCERT Solutions for Class 12 Chemistry Chapter 6: Haloalkanes and Haloarenes
- These solutions are in clear and easy language.
- Columns are used wherever necessary.
- Using these solutions, students will be able to address their doubts and conceptual mistakes.
Working out on more and more problems will help students to perform well in class 12 Board exams. NCERT Solution gives the answer to all these exercise in a clear, simple and straightforward way. Students can check their answers with the solutions provided. It is also another way coordination compounds class 12 NCERT solutions help students.
| Related Chapters |




 [Live Update] CUET PG Result 2025 @exams...
[Live Update] CUET PG Result 2025 @exams...
 UP, MP, CBSE Board Result 2025 Live Upda...
UP, MP, CBSE Board Result 2025 Live Upda...
 CUET UG Exam Date Sheet 2025 @cuet.nta.n...
CUET UG Exam Date Sheet 2025 @cuet.nta.n...






