Students who are looking for Class 12 Chemistry NCERT Solutions for Chapter 7 can refer to this article. Students will be provided detailed NCERT solutions for Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers. Students can find the NCERT Class 12 Chemistry notes PDF too.
This chapter of chemistry too begins with the IUPAC naming conventions for alcohols, phenols, and ethers. They also handle the production of alcohols, phenols, and ethers. They also investigate the characteristics of it.
NCERT Solutions For Class 12 Chemistry Chapter 7
NCERT Solution for Class 12 Chemistry Chapter 7 Alcohols, Phenols and Ethers is an Essential study material that is required for all students studying Class 12 Chemistry.
The Central Board of Secondary Education or CBSE is one of the most preferred educational boards in the country. The schools affiliated with CBSE are advised to follow the NCERT curriculum as well as the NCERT books.
After studying this chapter about Alcohol, phenols and ethers using the NCERT Solutions, students can learn how to write the name of alcohols, phenols and ethers according to the IUPAC system of nomenclature.
Detailed illustration of the differences between alcohols, phenols and ethers are elaborated in Class 12 Chemistry chapter 7 NCERT Solutions. After understanding the difference in nature, it becomes easier for students to understand all reactions involved in preparing alcohol, phenols and ethers from substances like Ketone, Aldehyde, Benzene, Sulphonic acid, Cumene respectively. The chapter on Alcohols, Phenols and Ethers in Chemistry comprises some of the most important concepts of advanced chemistry.
NCERT Solution Chemistry Class 12 Chapter 7 Alcohols, Phenols and Ethers
Q) Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
(ii) Oxidation of a primary alcohol to aldehyde.
(iii) Brominationofphenolto2,4,6-tribromophenol
(iv) Benzyl alcohol to benzoic acid.
(v) Dehydration of propan-2-oI to propene.
(vi) Butan-2-one to butan-2-oL .
Ans: (i) Acidified potassium dichromate or neutral/ acidic/ alkaline potassium permanganate.
(ii) Pyridinium chlorochromate (PCC), (C5H5NH)+ ClCrO3– in CH2Cl2
or Pyridinium dichromate (PDC),[(C5H5NH)2]2+Cr2O72-in CH2Cl2
(iii) Aqueous bromine, i.e., Br2/H2O.
(iv) Acidified or alkaline potassium permanganate.
(v) 85% H2S04 at 440 K.
(vi) Ni/H2 or NaBH4 or LiAlH4.
Q) Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Ans: The molecules of butane are held together by weak van der Waal’s forces of attraction while those of propanol are held together by stronger intermolecular hydrogen bonding.
Q) Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Ans: Alcohols can form hydrogen bonds with water and by breaking the hydrogen bonds already existing between water molecules. Therefore, they are soluble in water.
On the other hand, hydrocarbons cannot from hydrogen bonds with water and hence are insoluble in water.
Q) What is meant by hydroboration-oxidation reaction?
Ans: The addition of diborane to alkenes to form trialkyl boranes followed by their oxidation with alkaline hydrogen peroxide to form alcohols is called hydroboration-oxidation.
Q) While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Ans: O-nitrophenol is steam volatile due to chelation (intramolecular H – bonding) and hence can be separated by steam distillation from o-nitrophenol which is hot steam volatile because of intermolecular H-bonding.
Q) Explain why is ortho-nitrophenol more acidic than ortho-methoxyphenol?
Ans: Nitro (NO2) group is an electron withdrawing group while methoxy (OCH3) group is electron releasing in nature. The release of H+ ion is therefore, easier from o-nitrophenol while it is quite difficult from o-methoxyphenol. Apart from that, o-nitrophenoxide ion is stabilised due to resonance o-nitrophenol is steam volatile while p-nitrophenol is not. This is on account of intramolecular hydrogen bonding in the molecules of o-nitrophenol. As a result, its boiling point is less than that of p-nitrophenol in which the molecules are linked by intermolecular hydrogen bonding.
It is interesting to note that in the substituted phenols, the nature and position of the substituent influences the boiling point of phenol.
For example: .o-nitrophenol is steam volatile while p-nitrophenol is not. This is supported by the fact that the boiling point temperature of o-nitrophenol (100°C) is less than that of p-nitrophenol, (279°C). In o-nitrophenol, there is intramolecular hydrogen bonding in OH and NO2 groups placed in a adjacent positions. However, these are linked by intermolecular hydrogen bonding in the p-isomers. It is quite obvious that extra energy is needed to the-cleave the hydrogen bonds in the p-isomer. Consequently, its boiling point is more.
o-nitrophenol with lower boiling point is steam volatile while p-nitrophenol is not. This helps in the separation of the two isomers present in the liquid mixture. On passing steam, o-nitrophenol volatilises and its vapours rise along with steam and after condensation, collect in the receiver p-nitrophenol is left behind in the distillation flask. o-nitrophenol p-nitrophenol.
On the contrary, o-methoxyphenoxide is destabilised since the electron density on the negatively charged oxygen tends to increase due to the electron releasing tendency of the methoxy(OCH3) group.
In the light of the above discussion, we may conclude that o-nitrophenol is a stronger acid (pKa = 7-23) than o-methoxyphenol (pKa = 9.98)
Q) Explain how does the – OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Ans: Phenol may be regarded as a resonance hybrid of structures I-V, shown below.
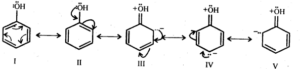
As a result of +R effect of the -OH group, the electron density in the benzene ring increases thereby facilitating the attack of an electrophile. In other words, presence of -OH group, activates the benzene ring towards electrophilic substitution reactions. Further, since the electron density is relatively higher at the two o-and one p-position, therefore electrophilic substitution occurs mainly at o-and p-positions.
Q) Explain the following with an example
(i) Kolbe’s reaction
(ii) Reimer – Tiemann reaction –
(iii) Williamson ether synthesis
(iv) Unsymmetrical ether
Ans: (i) Kolbe’s reaction: Sodium phenoxide when heated with C02 at 400K under a pressure of 4-7 atmospheres followed by acidification gives 2-hydroxybenzoic acid (salicylic acid) as the major product along with a small amount of 4-hydroxybenzoic acid.This reaction is called Kolbe’s reaction.
(ii) Reimer-Tiemann reaction: Treatment of phenol with CHC13 in presence of aqueous sodium or potassium hydroxide at 340 K followed by hydrolysis of the resulting product gives 2-hydroxybenzaldehyde (salicyialdehyde) as the major product. This reaction is called Reimer-Tiemann reaction.
(iii) Williamson’s ether synthesis: It involves the treatment of an alkyl halide with a suitable sodium alkoxide to obtain ethers. The sodium alkoxide needed for the purpose is prepared by the action of sodium on a suitable alcohol. In this reaction alkyl halide should primary. Secondary and tertiary halides will predominantly give an alkene.
(iv) Unsymmetrical ether: If the alkyl or aryl groups attached to the oxygen atom are different, ethers are called unsymmetrical ethers. For example, ethyl methyl ether, methyl phenyl ether, 4-chlorophenyl- 4-nitrophenyl ether, etc.
Q) Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Ans: Ethanol undergoes intermolecular H-bonding due to the presence of a hydrogen atom attached to the electronegative oxygen atom. As a result, ethanol exists as associated molecules.
Consequently, a large amount of energy is required to break these hydrogen bonds. Therefore, the boiling point of ethanol is higher than that of methoxymethane which does not form H-bonds.
Q) Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
(ii) Oxidation of a primary alcohol to aldehyde.
(iii) Brominationofphenolto2,4,6-tribromophenol
(iv) Benzyl alcohol to benzoic acid.
(v) Dehydration of propan-2-oI to propene.
(vi) Butan-2-one to butan-2-oL .
Ans: (i) Acidified potassium dichromate or neutral/ acidic/ alkaline potassium permanganate.
(ii) Pyridinium chlorochromate (PCC), (C5H5NH)+ ClCrO3– in CH2Cl2
or Pyridinium dichromate (PDC),[(C5H5NH)2]2+Cr2O72-in CH2Cl2
(iii) Aqueous bromine, i.e., Br2/H2O.
(iv) Acidified or alkaline potassium permanganate.
(v) 85% H2S04 at 440 K.
(vi) Ni/H2 or NaBH4 or LiAlH4.
Q) Explain the fact that in alkyl aryl ethers, alkoxy group :
(i) activates the benzene ring towards electrophilic substitution.
(ii) directs the incoming substituents towards ortho and para positions in the ring.
Ans:
(i) The alkoxy group (RO -) with lone electron pairs on the oxygen atom activates the ortho and para positions in the ring by + M (or + R) effect as shown below :
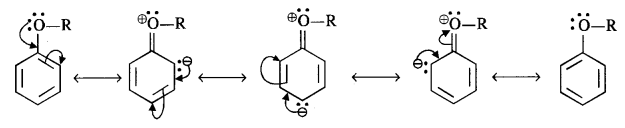
As the ortho and para positions in the ring become points of high electron density, the electrophiles prefer to attack these positions.
(ii) The alkoxy group directs the incoming group which is an electrophile towards the ortho and para positions in the ring. As a result, a mixture of isomeric products is formed.
| Related Chapters |










 RBSE Class 10 Model Paper 2026 Out, Down...
RBSE Class 10 Model Paper 2026 Out, Down...
 RBSE Class 12 Model Paper 2026 for Arts,...
RBSE Class 12 Model Paper 2026 for Arts,...
 SLAT Preparation 2026: Tips and Subject ...
SLAT Preparation 2026: Tips and Subject ...






