Table of Contents
Students preparing for the board exams should go through the NCERT solutions provided on this page for the Class 12 Chemistry chapter 12 to score good marks in the exam. The name of the NCERT class 12 chemistry chapter 12 is Aldehydes Ketones and Carboxylic Acids. As the chapter in itself consists of three different carbon group, it is important to revise it carefully by going through the notes provided below. After studying, students can attempt the NCERT questions and match their answers with the ones given on this page.
NCERT Solutions for Class 12 Chemistry Chapter 12
The twelfth chapter deals with the Aldehydes, Ketones and Carboxylic Acids. These compounds contains carbon-oxygen double bond which are known as carbonyl compounds.
In Aldehydes, the carbonyl group is attached to a single hydrogen atom and a single alkyl or aryl group, Ketones, the carbonyl group is attached to either two alkyls or aryl groups or both the groups.
Students should study this topic with greater attention because this chapter is a vital part of chemistry Class 12. However, while preparing this chapter, students might face many difficulties but with right/ proper guidance, they might prepare this chapter and score god marks in exams.
Organic Chemistry is scoring part of the syllabus. While preparing for exams students can take help from this solution for qualitative preparation.
Students can easily download these PDF as per their convenience. The answers are elaborately explained along with several illustrations to help students understand the concepts better.
NCERT Solutions Chemistry Class 12 Chapter 12
Q) Name the following compounds according to IUPAC system of nomenclature:
i.) CH3CH(CH3)CH2CH2CHO
ii.) CH3CH2COCH(C2H5)CH2CH2Cl
iii.) CH3CH=CHCHO
iv.) CH3COCH2COCH3
v.) CH3CH(CH3)CH2C(CH3)2COCH3
vi.) (CH3)3CCH2COOH
vii.) OHCC6H4CHO-p
Answer:
i.) 4-Methylbutanal
ii.) p-Nitropropiophenone
iii.) p-Methylbenzaldehyde
iv.) 4-Methylpent-3-en-2-one 4-chloropentan-2-one
v.) 3-Bromo-4-phenylpentanoic acid
vi.) p, p’-Dihydroxybenzophenone
vii.) Hex-2-en-4-ynoic acid.
Q) Write the IUPAC names of the following ketones and aldehydes. Wherever possible give also common names.
(i)CH3CO(CH2)4CH3
(ii) CH3CH2CHBrCH2CH(CH3)CHO
(iii) CH3(CH2)5CHO
(iv) Ph-CH=CH-CHO
(iv) PhCOPh
Answer:
(i) IUPAC name: Heptan-2-one
Common name: Methyl n-propyl ketone
(ii) IUPAC name: 4-Bromo-2-methylhexanal
Common name: (γ-Bromo- α-methyl-caproaldehyde)
(iii) IUPAC name: Heptanal
(iv) IUPAC name: 3-phenylprop-2-enal
Common name: β-Phenolyacrolein
(v) IUPAC name: Diphenylmethanone
Common name: Benzophenone
Q) Arrange the following compounds in increasing order of the their property indicated:
i.) Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone(reactivity towards HCN)
ii.) CH3CH2CH(Br)COOHJ, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)
iii.) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Answer:
(i) When HCN reacts with a compound, the attacking species is a nucleophile, CN – . Therefore, as the negative charge on the compound increases, its reactivity with HCN decreases. In the given compounds, the +I effect increases as shown below. It can be observed that steric hindrance also increases in the same
Hence, the given compounds can be arranged according to their increasing reactivities toward HCN as:
Di-tert-butyl ketone < Methyl tert-butyl ketone < Acetone < Acetaldehyde
(ii) After losing a proton, carboxylic acids gain a negative charge as shown:
R-COOH à R-COO- + H+
Now, any group that will help stabilize the negative charge will increase the stability of the carboxyl ion and as a result, will increase the strength of the acid. Thus, groups having +I effect will decrease the strength of the acids and groups having – I effect will increase the strength of the acids. In the given compounds, – CH3group has +I effect and Br – group has – I effect. Thus, acids containing Br – are stronger.
Now, the +I effect of isopropyl group is more than that of n-propyl group. Hence, (CH3)2CHCOOH is a weaker acid than CH3CH2CH2COOH.
Also, the – I effect grows weaker as distance increases. Hence, CH3CH(Br)CH2COOH is a weaker acid than CH3CH2CH(Br)COOH.
Hence, the strengths of the given acids increase as:
(CH3)2CHCOOH < CH3CH2CH2COOH < CH3CH(Br)CH2COOH < CH3CH2CH(Br)COOH
(iii) As we have seen in the previous case, electron-donating groups decrease the strengths of acids, while electron-withdrawing groups increase the strengths of acids. As methoxy group is an electron-donating group, 4-methoxybenzoic acid is a weaker acid than benzoic acid. Nitro group is an electron-withdrawing group and will increase the strengths of acids. As 3,4-dinitrobenzoic acid contains two nitro groups, it is a slightly stronger acid than 4-nitrobenzoic acid. Hence, the strengths of the given acids increase as:
4-Methoxybenzoic acid < Benzoic acid < 4-Nitrobenzoic acid < 3,4-Dinitrobenzoic acid
Q) Give simple chemical tests to distinguish b/w the following pairs of compounds.
i.) Propanal and propanone
ii.) Acetophenone and Benzophenone
iii.) Phenol and Benzoic acid
iv.) Benzoic acid and Ethyl benzoate
v.) Pentan-2-one and Pentan-3-one
vi.) Benzaldehyde and Acetophenone
vii.) Ethanol and Propanal
Answer:
(i) Propanal and propanone can be distinguished by the following tests.
(a) Tollen’s test
Propanal is an aldehyde. Thus, it reduces Tollen’s reagent. But, propanone being a ketone does not reduce Tollen’s reagent.
(b) Fehling’s test
Aldehydes respond to Fehling’s test, but ketones do not.
Propanal being an aldehyde reduces Fehling’s solution to a red-brown precipitate of Cu2O, but propanone being a ketone does not.
(c) Iodoform test:
Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom respond to iodoform test. They are oxidized by sodium hypoiodite (NaOI) to give iodoforms. Propanone being a methyl ketone responds to this test, but propanal does not.
(ii) Acetophenone and Benzophenone can be distinguished using the iodoform test.
Iodoform test:
Methyl ketones are oxidized by sodium hypoiodite to give yellow ppt. of iodoform. Acetophenone being a methyl ketone responds to this test, but benzophenone does not.
(iii) Phenol and benzoic acid can be distinguished by ferric chloride test.
Ferric chloride test:
Phenol reacts with neutral FeCl3 to form an iron-phenol complex giving violet colouration.
But benzoic acid reacts with neutral FeCl3 to give a buff coloured ppt. of ferric benzoate.
(iv) Benzoic acid and Ethyl benzoate can be distinguished by sodium bicarbonate test.
Sodium bicarbonate test:
Acids react with NaHCO3 to produce brisk effervescence due to the evolution of CO2 gas.
Benzoic acid being an acid responds to this test, but ethylbenzoate does not.
(v) Pentan-2-one and pentan-3-one can be distinguished by iodoform test.
Iodoform test:
Pentan-2-one is a methyl ketone. Thus, it responds to this test. But pentan-3-one not being a methyl ketone does not respond to this test.
(vi) Benzaldehyde and acetophenone can be distinguished by the following tests.
(a) Tollen’s Test
Aldehydes respond to Tollen’s test. Benzaldehyde being an aldehyde reduces Tollen’s reagent to give a red-brown precipitate of Cu2O, but acetophenone being a ketone does not.
(b) Iodoform test
Acetophenone being a methyl ketone undergoes oxidation by sodium hypoiodite (NaOI) to give a yellow ppt. of iodoform. But benzaldehyde does not respond to this test.
(vii) Ethanal and propanal can be distinguished by iodoform test.
Iodoform test
Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom responds to the iodoform test. Ethanal having one methyl group linked to the carbonyl carbon atom responds to this test. But propanal does not have a methyl group linked to the carbonyl carbon atom and thus, it does not respond to
Q) Describe the following:
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol aondensation
(iv) Decarboxylation
Answer:
(i) Acetylation:
The introduction of an acetyl functional group into an organic compounds is known as acetylation. It is usually carried out in the presence of a base such as pyridine, dimethylaniline, etc. This process involves the substitution of an acetyl group for an active hydrogen atom. Acetyl chloride and acetic anhydride are commonly used as acetylating agents.
For example, acetylation of ethanol produces ethyl acetate.
(ii) Cannizzaro reaction:
The self oxidation – reduction reaction of aldehydes having no α – hydrogen’s on treatment with concentrated alkalis is known as the cannizzaro reaction. In this reaction, two molecules of aldehydes participates where one is reduced to alcohol and the other is oxidised to carboxylic acid.
For example, when ethanol is treated with concentrated potassium hydroxide, ethanol and potassium ethanoate are produced.
(iii) Cross – aldol condensation:
When aldol condensation is carried out between two different aldehydes, or two different ketones, or an aldehyde and a ketones, then the reaction is called a cross – aldol condensation. If both the reactants contain α – hydrogen, four compounds are obtained as products.
For example, ethanol and propanal react to give four products.
(iv) Decarboxylation:
Decarboxylation refers to the reaction in which carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with soda – lime.
Decarboxylation also takes place when aqueous solutions of alkali metal salts of carboxylic acids are electrolyzed. This electrolytic process is known as Kolbe’s electrolysis.
Q) Give plausible explanation for each of the following:
Cyclohexanone forms cyanohydrin in good yield nut 2,2,6-trimethylcyclohexanone does not. There are two –NH2 groups in semicarbazide. However, only one is involved in the formation of semicarbazones. During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the water or the ester should be removed as soon as it is formed.
Answer: In this case, the nucleophile CN- cab easily attack without any steric hindrance. However, in the case of 2, 2, 6- trimethylcyclohexanone, methyl groups at α-positions offer steric hindrance and a s result, CN- cannot attack effectively.
For this reason, it does not form a cyanohydrin.
Semicarbazide undergoes resonance involving only one of the two –NH2 groups, which is attached directly to the carbonyl – carbon atom.
Therefore, the electron density on –NH2 group involved in the resonance also decreases. As a result, it cannot act as a nucleophile. Since, the other –NH2 group is not involved in resonance; it can act as nucleophile and can attack carbonyl – carbon atoms of aldehydes and ketones tom produce semicarbazones.
Ester along with water is formed reversibly from a carboxylic acid and an alcohol in presence of an acid.
If either water or ester is not removed as soon as it is formed, then it reacts to give back the reactants as the reaction is reversible. Therefore, to shift the equilibrium in the forward direction i.e., to produce more ester, either of the two should be removed.
Q) An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce tollen’s reagent nut forms an addition compound with sodium hydroigensulphite and give positive idoform test. On vigorous oxidation it gives ethanoic and propanoic acid.
Answer:
% of carbon = 69.77 %
% of hydrogen = 11.63 %
% of oxygen = {100 – (69.77 + 11.63)}%
= 18.6 %
Thus, the ratio of the number of carbon, hydrogen, and oxygen atoms in the organic compound can be given as:
= 5.81:11.63:1.16
= 5:10:1
Therefore, the empirical formula of the compound is C5H10O. Now, the empirical formula mass of the compound can be given as:
5 × 12 + 10 ×1 + 1 × 16
= 86
Molecular mass of the compound = 86
Therefore, the molecular formula of the compound is given by C5H10O.
Since the given compound does not reduce Tollen’s reagent, it is not an aldehyde. Again, the compound forms sodium hydrogen sulphate addition products and gives a positive iodoform test. Since the compound is not an aldehyde, it must be a methyl ketone.
The given compound also gives a mixture of ethanoic acid and propanoic acid.
Hence, the given compound is Pentan-2-one.
Q) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
Answer:
i.) Phenoxide ion has non-equivalent resonance structures in which the negative charge is at the less electronegative carbon atom whereas in case of carboxylate ion both the resonating structures are equivalent.
ii.) The negative charge is delocalised over two electronegative oxygen atoms in carboxylate ion whereas in phenoxide ion the negative charge less effectively delocalises over one oxygen atom and less electronegative carbon atoms. So the carboxylate ion is more resonance stabilised than phenoxide ion. Thus, the release of proton from carboxylic acid is much easier than from phenol. Hence, carboxylic acid is a stronger acid than phenol.
Q) Arrange the following carbonyl compounds in increasing order of their reactivity in nucleophilic addition reactions :
(a) Ethanal, propanal, propanone, butanone
(b) Benzaldehyde, p-tolualdehyde, p-nitrobenzaldehyde, acetophenone
Answer: (a) The increasing order of reactivity of the carbonyl compounds towards nucleophilic addition reactions is :
butanone < propanone < propanal < ethanal
The reactivity is based upon two factors. These are: steric factors and electronic factors.
(b) The increasing order of reactivity is :
acetophenone < p-tolualdehyde < benzaldehyde < p-nitrobenzaldehyde
Explanation: Acetophenone being a ketone is the least reactive towards nucleophilic addition. All others are aldehydes. Among them, p-tolualdehyde is less reactive than benzaldehyde because CH3 group present at the para position w.r.t. -CHO group will increase the electron density on the carbonyl carbon atom due to hyper conjugation effect. As a result, the nucleophile attack occurs to lesser extent as compared to benzaldehyde.
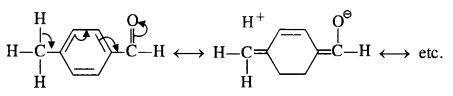
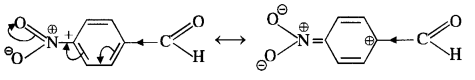
In p-nitrobenzaldehyde, the nitro group has an opposing effect. It is electron withdrawing in nature due to -I effect as well as -R effect. The electron density on the carbonyl carbon atom decreases and this favours the nucleophile attack.




 NCERT Solutions for Class 12 Chemistry C...
NCERT Solutions for Class 12 Chemistry C...
 Class 12 Chemistry Chapter 9 NCERT Solut...
Class 12 Chemistry Chapter 9 NCERT Solut...
 Class 12 Chemistry Chapter 8 NCERT Solut...
Class 12 Chemistry Chapter 8 NCERT Solut...






