Class 12 Chemistry NCERT Solutions: Adda247 offers NCERT Solutions for Chapter 14 of Class 12 Chemistry named Biomolecules. The NCERT Solutions offered here will improve students’ understanding and also propose different approaches for teachers to tackle specific questions.
In this chapter, students explore biomolecules such as glucose, fructose, starch, and glycogen. They discover the structures, classifications, and characteristics of carbohydrates and proteins. They also study the reactions of certain biomolecules.
NCERT Solutions for Class 12 Chemistry Chapter 14: Biomolecules
NCERT includes chapters of biomolecules in the Class 12 board exams syllabus. Chapter 14 is elaborated in NCERT Solutions for Class 12 Chemistry and one of the most interesting chapters of the Class 12 syllabus.
It deals with the fact that all living beings are made up of non-living atoms and molecules. These molecules are referred to as Biomolecules. Class 12 Chemistry Chapter 14 NCERT Solutions deals with the subject of biomolecules in a very comprehensive manner and it gives a student a complete overview of the chapter and also a solution to all questions asked in the exercise.
Chapter 14 of the class 12 syllabus about Biomolecules deals with the complex subject of chemicals produced by a living organism to sustain life, promote growth and reproduce. It is an important part of the class 12 Chemistry syllabus as it is very basic of Biochemistry. Hence, it needs to be prepared well. NCERT Class 12 Chemistry Biomolecules PDF downloaded gives a complete overview regarding the Chapter.
Chapter 14 of the class 12 syllabus comprises the concept of Biomolecules. It explains that all living beings grow to sustain and reproduce. But all living organisms are made up of non-living atoms and molecules and all changes take place through a chemical reaction in a living organism. The NCERT Solutions of Biomolecules Class 12 give a comprehensive view of chemical change that goes on inside a living being.
Subtopics of chapter 14
- Carbohydrates
- Proteins
- Enzymes
- Vitamins
- Nucleic acid
- Hormones
An important aspect of Biomolecules Class 12 is the importance of Carbohydrates and proteins in our food.
NCERT Solutions Chemistry Class 12 Chapter 14
Q) What are monosaccharides?
Answer: Monosaccharides (from Greek monos: single, sacchar: sugar), also called simple sugars, are the simplest form of sugar and the most basic units (monomers) of carbohydrates.[1] The general formula is CnH2nOn, albeit not all molecules fitting this formula (e.g. acetic acid) are carbohydrates. [2] They are usually colorless, water-soluble, and crystalline solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste.
Q) Give examples of monosaccharides.
Examples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose and lactose) and polysaccharides (such as cellulose and starch). Each carbon atom that supports a hydroxyl group is chiral, except those at the end of the chain. This gives rise to a number of isomeric forms, all with the same chemical formula. For instance, galactose and glucose are both aldohexoses, but have different physical structures and chemical properties.
Q) What is the role of monosaccharides in Metabolism?
The monosaccharide glucose plays a pivotal role in metabolism, where the chemical energy is extracted through glycolysis and the citric acid cycle to provide energy to living organisms. Some other monosaccharides can be converted in the living organism to glucose.
Q) What are reducing sugars?
Answer: Reducing sugars are carbohydrates that reduces Fehling’s solution and Tollen’s reagent. All monosaccharides and disaccharides, excluding sucrose, are reducing sugars.
Q) Write two main functions of carbohydrates in plants.
Answer: The two main functions of carbohydrates in plants are:
i.) Polysaccharides such as starch serve as storage molecules.
ii.) Cellulose, a polysaccharide, is used to build the cell wall.
Q) What us glycogen? How is it different from starch.
Answer: Glycogen is a carbohydrate. In animals, carbohydrate are stored as glycogen. Starch is a carbohydrate consisting of two component – amylose and amylopectin. However, glycogen consists of only one component whose structure is similar is similar to amylopectin. Also, glycogen is more branched than amylopectin.
Q) Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Answer: The reactions of D-glucose that cannot be explained by its open chain structure are:
(1) Aldehydes give 2, 4-DNP test, Schiff’s test, and react with NaHSO4to form the hydrogen sulphite addition product. However, glucose does not undergo these reactions.
(2) The pentaacetate of glucose does not react with hydroxylamine. This indicates that a free -CHO group is absent from glucose.
(3) Glucose exists in two crystalline forms – ∝ and β. The ∝-form (m.p. = 419 K) crystallises from a concentrated solution of glucose at 303 K and the β-form (m.p = 423 K) crystallises from a hot and saturated aqueous solution at 371 K. This behaviour cannot be explained by the open chain structure of glucose.
Q) What are essential and non essential amino acids? Give two examples of each type.
Answer: Essential amino acids are required by the human body, but they cannot by synthesized in the body. They must be taken though food. For example, valine and leucine. Non-essential.
Q) Define the following as related to proteins:
- Peptide linkage
- Primary structure
- Denaturation
Answer:
- A peptide linkage is an amide (—CO— NH —) linkage formed between —COOH group of one amino acid and —NH2 group of other a-amino acid by loss of a water molecule.
- The specific sequence in which various a-amino acids present in a protein are linked to one another is called its primary structure. Any change in its primary structure creates a new protein
- Denaturation of Proteins: When a protein in its native form is subjected to a change, such as change in temperature or change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein. During denaturation, 2° and 3° structures are destroyed but 1° structures remain intact, e.g., coagulation of egg while on boiling, curdling of milk, etc.
Q) What are nucleic acids? Mention their two important functions.
Answer: Nucleic acids are biomolecules found in the nuclei of all living cells, as one of the constituents of chromosomes. There are mainly two types of nucleic acids – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleic acids are also known as polynucleotides as they are long-chain polymers of nucleotides.
Two main functions of nucleic acids are:
(i) DNA is responsible for the transmission of inherent characters from one generation to the next. This process of transmission is called heredity.
(ii) Nucleic acids (both DNA and RNA) are responsible for protein synthesis in a cell. Even though the proteins are actually synthesised by the various RNA molecules in a cell, the message for the synthesis of a particular protein is present in DNA.
Q) Differentiate between globular and fibrous proteins.
Answer:
| Fibrous protein | Globular protein |
| It is fiber-like structure formed by the polypeptide chain. These proteins are held together by strong hydrogen and disulphide bonds. | The polypeptide chain in this protein is folded around itself, giving rise to a spherical structure. |
| It is usually insoluble in water. | It is usually soluble in water. |
| Fibrous proteins are usually used for structural purposes. For example, keratin is present in nails and hair, collagen in tendons, and myosin in muscles. | All enzymes are globular proteins. Some hormones such as insulin are also globular proteins. |
Q) Write the important structural and functional difference between DNA and RNA.
Answer:
The structural difference between DNA and RNA are as follows:
| DNA | RNA |
| The sugar present in DNA molecules is -D-2-deoxyribose. | The sugar present in RNA molecules is -D-ribose. |
| DNA contains thymine. It does not contain uracil. | RNA contains uracil. It does not contain thymine. |
| The helical structure of DNA is double-stranded. | The helical structure of RNA is single stranded. |
The functional difference between DNA and RNA are as follows:
| DNA | RNA |
| DNA is the chemical basis of heredity. | RNA is not responsible for heredity. |
| DNA molecules do not synthesize proteins, but transfer coded message for the synthesis of proteins in the cell. | Proteins are synthesized by RNA molecules in the cells. |
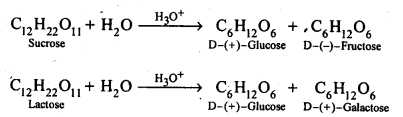
Q) What are the hydrolysis products of (i) sucrose, and (ii) lactose?
Answer: Both sucrose and lactose are disaccharides. Sucrose on hydrolysis gives one molecule each of glucose and fructose but lactose on hydrolysis gives one molecule each of glucose and galactose.
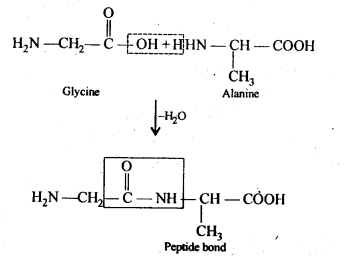
Q) What are essential and non-essential amino acids? Give two examples of each type.
Answer: α-Amino acids which are needed for good health and proper growth of human beings but are not synthesized by the human body are called- essential amino acids. For example, valine, leucine, phenylalanine, etc. On the other hand, α-amino acids which are needed for health and growth of human beings and are synthesized by the human body are called non-essential amino acids. For example, glycine, alanine, aspartic acid etc.
Q) Define the following as related to proteins:
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation
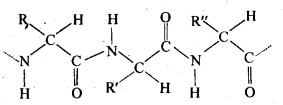
Ans: (i) Peptide bond: Proteins are condensation polymers of α-amino acids in which the same or different α-amino acids are joined by peptide bonds. Chemically, a peptide bond is an amide linkage formed between – COOH group of one α-amino acid and -NH-, group of the other α-amino acid by lo;ss of a molecule of water. For example,
(ii) Primary structure: Proteins may contain one or more polypeptide chains. Each . polypeptide chain has a large number of α-amino acids which are linked to one another in a specific manner. The specific sequence in which the various amino acids present in a protein linked to one another is called its primary structure. Any change in the sequence of α-amino acids creates a different protein.
(iii) Denaturation: Each protein in the biological system has a unique three-dimensional structure and has specific biologicalactivity. This is called native form of a protein. When a protein in its native form is subjected to a physical change such as change in temperature or a chemical change like change in pH, etc., hydrogen bonds gets broken. As a result, soluble forms of proteins such as globular proteins undergo coagulation or precipitation to give fibrous proteins which are insoluble in water. This coagulation also results in loss of biological activity of the proteins and this loss in biological activity, is called denaturation. During denaturation, 2° and 3° structures of proteins are destroyed but 1° structure remains intact.
The most common example of denaturation of proteins is the coagulation of albumin present in the white of an egg. When the egg is boiled hard, the soluble globular protein present in it is denatured and is converted into insoluble fibrous protein.










 NCERT Solutions for Class 12 Chemistry C...
NCERT Solutions for Class 12 Chemistry C...
 Class 12 Chemistry Chapter 9 NCERT Solut...
Class 12 Chemistry Chapter 9 NCERT Solut...
 Class 12 Chemistry Chapter 8 NCERT Solut...
Class 12 Chemistry Chapter 8 NCERT Solut...

