The NCERT solutions for class 12 chemistry will prove to be helpful for students going to take the board exam in the coming month of February-March 2205. On this page, we have provided students with the NCERT solutions for class 12 chemistry chapter 15 Polymers.
This chapter focuses on polymer science, covering the different types of polymers, including addition or condensation, linear or crosslinked, along with their reactions and characteristics. This chapter additionally discusses several renowned polymers and their uses.
NCERT Solutions For Class 12 Chemistry Chapter 15 Polymers
Polymers are large macromolecules made from monomers through a process called Polymerization. In Polymers Class 12 NCERT PDF students will learn about the different classes of Polymers and the different types of Polymerization processes.
Polymer, any of a class of natural or synthetic substances composed of very large molecules, called macromolecules, that are multiples of simpler chemical units called monomers. Polymers make up many of the materials in living organisms, including, for example, proteins, cellulose, and nucleic acids.
Polymers are used in almost every area of modern living. Grocery bags, soda and water bottles, textile fibers, phones, computers, food packaging, auto parts, and toys all contain polymers. Even more-sophisticated technology uses polymers.
There are many types of polymers including synthetic and natural polymers.
Synthetic polymers are human-made polymers. They can be classified into four main categories: thermoplastics, thermosets, elastomers, and synthetic fibers. They are commonly found in a variety of consumer products.
Natural polymers include silk, hair, proteins and DNA, while synthetic (man-made) polymers include polyethylene, polypropylene, and polyester. Addition polymerization is the creation of a polymer by adding together monomers in a repeating pattern, with no resulting by-product.
Polymers are more resistant to chemicals than their metal counterparts. Polymer parts do not require post-treatment finishing efforts, unlike metal. Polymer and composite materials are up to ten times lighter than typical metals. Polymers are naturally radar-absorbent as well as thermally and electrically insulating.
The chapter then goes on to explain the different categories of monomers. Following these, the chapter highlights the preparation of different types of Polymers and their properties.
After this, students will learn about the use of polymers in everyday life. They can make use of Polymers Class 12 NCERT Solutions to further increase knowledge and expertise.
NCERT Solutions of Chemistry Chapter 15
Candidates should revise the important NCERT questions and their solutions from chapter 15 below.
Q) Explain the terms polymer and monomer.
Answer: Polymers are high molecular mass macromolecules composed of repeating structural units derived from monomers. Polymers have a high molecular mass. In polymers, various monomer units are joined by strong covalent bonds. Polymers can be natural as well as synthetic. Polythene, rubber, and nylon6,6 are examples of polymers. Monomers are simple, reactive molecules that combine with each other in large numbers through covalent bonds to give rise to polymers. For example ethene, propene, vinyl chloride.
Q) What are natural and synthetic polymers? Give two examples of each type.
Answer: Natural polymers are polymers that are found in nature. They are formed by plants and animals. Examples include proteins, cellulose, starch, etc. synthetic polymers are polymers made by human beings. Examples include plastics, synthetic fibers, synthetic rubber, etc.
Q) Distinguish between the terms homopolymer and copolymer and give an example of each.
Answer:
| Homopolymer | Copolymer |
| The polymers that are formed by the polymerization of a single monomer ate known as homopolymers. In other words, the repeating units of homopolymers are derived only from one monomer. | The polymers whose repeating units are derived from two types of monomers known as copolymers. |
| For example – polythene is homopolymer of ehtene. | For example – buna-s, is a polymer of 1,3-butadiene and styrene. |
Q) How do you explain the functionality of a monomer?
Answer: The functionality of a monomer is the number of binding sites that is/are present in that monomer.
For example, the functionality of monomers such as ethene and propene is one and that of 1,3-butadiene and adipic acid is two.
Q) Define the term polymerisation.
Answer: Polymerization is the process of forming high molecular mass macromolecules, which consists of repeating structural units derived from monomers. In a polymere, various monomer units are joined by strong bonds.
Q) Is (NH-CHR-CO)n, a homopolymer or completely?
Answer: (NH-CHR-CO), is homopolymer, because it is obtained from a single monomer unit,
NH2-CHR-COOH
Q) In which classes, the polymers are classified on the basis of molecular forces?
Answer: On the basis of magnitude of intermolecular forces present in the polymers, they are classified into the following groups;
- Elastomers.
- Fibers.
- Thermoplastic polymers.
- Thermosetting polymers.
Q) How can you differentiate between addition and condensation polymerisation?
Answer:
| Addition polymerization | Condensation polymerization |
| Monomers must have either a double bond or triple bond. | Monomers must have two similar or different functional groups. |
| Produces no by- products. | By – products such as ammonia water and HCl are produced. |
| Addition of monomers result in polymer . | Condensation of polymers result in monomers. |
| The molecular weight of the resultant polymers is a multiple of monomer’s molecular weight. | The molecular weight of the resultant polymers is not a multiple of monomer’s molecular weight. |
| Lewis acids or base, radicals are catalyst in addition polymerization. | The catalyst in condensation polymerisation are catalyst in condensation polymerization. |
| Common examples are PVC, Teflon. | Common examples are nylon, silicon etc. |
Q) Define thermoplastic and thermosetting polymers with two examples of each.
Answer: Thermoplastic polymers are linear long chain polymers, which can be repeatedly softened and hardened on heating. Hence, they can be modified again and again.
Examples include polythene, polystyrene.
Thermosetting polymers are cross-linked or heavily branched polymers which get hardened during the moulding process. These plastics cannot be softened again on heating. Examples of thermosetting plastics include Bakelite, urea – formaldehyde.
Q) Discuss the main purpose of vulcanisation of rubber.
Answer: Natural rubber though useful has some problems associated with its use. These limitations are discussed below:
- Natural rubber is quite soft and sticky at room temperature. At elevated temperatures (> 335 K), it becomes even softer. At low temperatures (< 283 K), it becomes brittle. Thus, to maintain its elasticity, natural rubber is generally used in the temperature range of 283 K-335 K.
- It has the capacity to absorb large amounts of water.
- It has low tensile strength and low resistance to abrasion.
- It is soluble in non-polar solvents.
- It is easily attacked by oxidizing agents.
Vulcanization of natural rubber is done to improve upon all these properties. In this process, a mixture of raw rubber with sulphur and appropriate additive is heated at a temperature range between 373 K and 415 K.
This is a slow process, therefore some additives like zinc oxide etc. are used to accelerate the process. During this process, sulphur cross links are formed which makes rubber hard, tough with greater tensile strength .The vulcanized rubber has excellent elasticity, low water absorption, resistance to oxidation & organic solvents.
Q) Write the monomers used for getting the following polymers.
- Polyvinyl chloride
- Teflon
- Bakelite
Answer:
- Vinyl chloride (CH2=CHCl)
- Tetreafluoroethylene (CF2=CF2)
- Formaldehyde (HCHO) and phenol (C6H5OH)
Q) Explain the term copolymerisation and give two examples.
Answer: When two or more different monomers are allowed to polymerise together the product formed is called a copolymer, and the process in called copolymerisation. Example, Buna-S and Buna-N. Buna- S is a copolymer of 1, 3- butadiene and styrene while Buna-N is a copolymer of 1,3-butadiene and acrylonitrile.
Q) Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Answer:
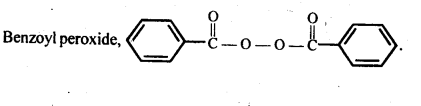
Q) Define thermoplastics and thermo setting polymers with two examples of each
Answer: Thermoplastics polymers are linear polymer which can be repeatedly melted and moulded again and again on heating without any change in chemical composition and mechanical strength. Examples are polythene and polypropylene.
Thermosetting polymers, on the other hand, are permanently setting polymers. Once on heating in a mould, they get hardened and set, and then cannot be softened again. This hardening on heating is due to cross- linking between different polymeric chains to give a three dimensional network solid. Examples are bakelite, melamine-formaldehyde polymer etc.











 NCHMCT JEE 2026: Exam Date, Online Regis...
NCHMCT JEE 2026: Exam Date, Online Regis...
 CUET PG 2026 Registration Started at exa...
CUET PG 2026 Registration Started at exa...
 CLAT Result 2026 Release Date, How to Do...
CLAT Result 2026 Release Date, How to Do...






