Table of Contents
Net Ionic Equation: Net ionic equations are an important aspect of chemistry as they largely depict what changes in a chemical process. The net ionic equation describes the ions that occur in an aqueous medium. We employ net ionic equations to highlight the molecules that shift during the process. Because they are the only molecules contained in the equation, it is relatively easy to identify the active molecules in a process. For example, Salts dissolve in polar solvents like water and then exist as cations and anions.
Net Ionic Equation
A net ionic equation is defined as an equation that displays just the molecules or ions that participate proactively in the reaction or those that change. The net ionic equation identifies molecules that only change chemically. Ions that emerge on both sides of the equation remain constant and are so classified as spectator ions. The spectator ions are not included in the net ionic equation. Many equations have been encountered, the majority of which are condensed equations in which all reactants and products are represented as electrically neutral compounds and molecules.
Net Ionic Equation Definition
To learn the Net Ionic Equation Definition better you must understand first the definition of Molecular equation, complete ionic equation.
Molecular equation
- A molecular equation is also known as a balanced equation. Any ionic compounds or acids can be expressed as neutral substances in a molecular equation by their chemical formulae. Following the formula, brackets denote the state of each substance.
- Suppose the reaction that takes place between AgNo3 and Nacl. When aqueous AgNO3 and NaCl solutions are combined, solid and aqueous NaNO3 are produced. We can create a balanced molecular equation for the reaction employing this information:
AgNO3(aq) + NaCl(aq) = AgCl(s) + NaNO3(aq) - However, if we were to magnify the contents of the reaction beaker, we could not see actual molecules of AgNO3, NaCl, or NaNO3. Because AgNO3, NaCl, and NaNO3 are soluble ionic substances, they dissolve in water into their constituent ions. NaCl, for example, dissociates into one Na+ ion for every CI ion; these ions are stabilized by ion-dipole interactions with the neighboring water molecules.
Complete Ionic Equation
- To obtain the complete ionic equation, we may redefine the soluble ionic compounds as disintegrating ions using the molecular formula:
Ag+ (aq) + NO3 (aq) +Na+ (aq) + Cl(aq) → AgCl(s) + Na+ (aq) + NO3 (aq)
- Although AgCl is insoluble in water, we did not change the depiction of AgCl(s). In general, soluble ionic compounds, strong acids, and strong bases must be divided into their constituent ions in an entire ionic equation, whereas insoluble salts and weak acids should remain together.
- When we examine our entire ionic equation, we can see that NO3 (aq) and Na+ (aq) can be found on both sides of the reaction arrow. During a process, ionic species that remain intact in this manner are referred to be spectator ions.
- Because spectator ions occur on both sides of the equation, we may cancel them out in the same way as equal terms on either side of an equation in math can be canceled out:
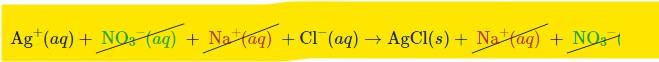
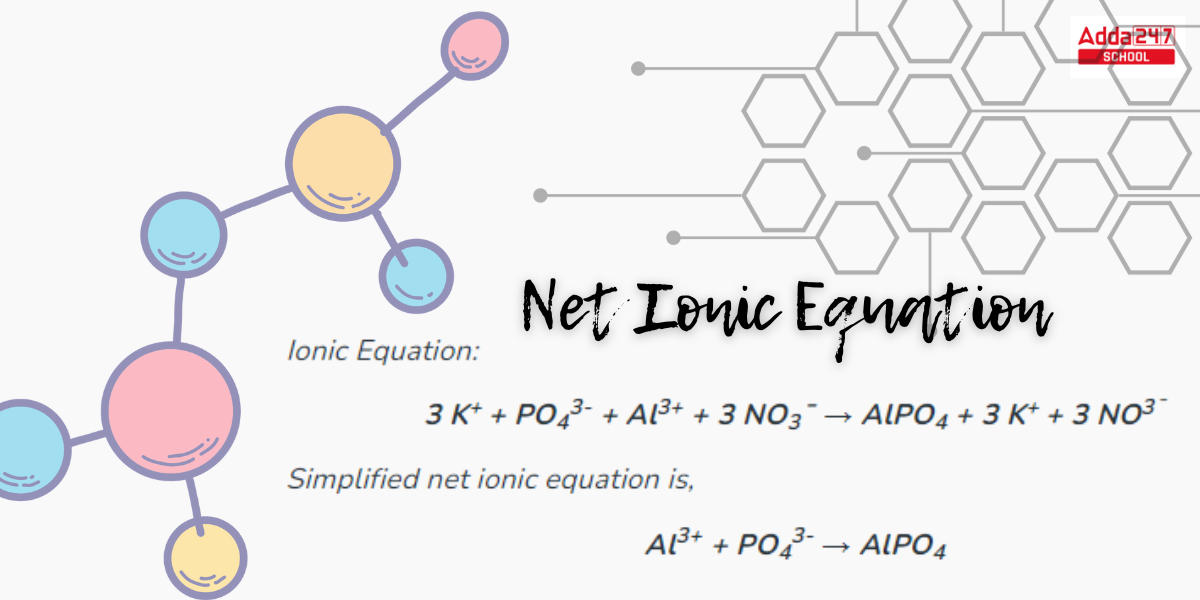
Net Ionic Equation
- This results in the net ionic equation, which only indicates the species actually participating in the chemical reaction:
AgCl(s) = Ag(aq) + Cl(aq). - This net ionic equation indicates that solid silver chloride is formed from dispersed Ag+ and Cl ions, despite their source. In comparison, the complete ionic equation describes all of the ions present in the solution throughout the reaction, whereas the molecular equation describes the ionic molecules utilized as sources of Ag+ and Cl during the process.
- A net ionic equation is written with the fewest possible integer values for the stoichiometric coefficients per convention. That indicates that, in order to obtain the final form of the net ionic equation, we might require dividing all of its coefficients by a common divisor.
Net Ionic Equation Formula
Let’s say we have the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl) to form silver chloride (AgCl) and sodium nitrate (NaNO3).
- Write the balanced molecular equation: AgNO3 + NaCl -> AgCl + NaNO3
- Break down the reactants and products into their ions: Ag+ + NO3- + Na+ + Cl- -> Ag+ + Cl- + Na+ + NO3-
- Identify the spectator ions (ions that appear on both sides): Ag+ and NO3-
- Write the net ionic equation without the spectator ions: Cl- + Na+ -> AgCl
The net ionic equation for this reaction is Cl- + Na+ -> AgCl.
This equation represents the essential chemical change that occurs when silver nitrate and sodium chloride react to form silver chloride.
How to write a Net Ionic Equation?
Students can calculate the Net Ionic Formula by following the step-by-step process mentioned below.
Stage 1: Recognise the key differences between ionic and molecular compounds. The first step in developing a net ionic equation is to identify the ionic compounds involved in the process. Ionic chemicals are those that ionize in an aqueous solution due to their charge. Molecules are substances that are never charged. Because they are formed between two nonmetallic substances, they are sometimes referred to as covalent compounds.
Stage 2: Determine the solubility of a chemical. Because not all ionic compounds are soluble in aqueous solution, they do not dissociate into individual ions. You must first determine the solubility of each of the molecules before proceeding with the remaining equation.
Stage 3: Analyse a chemical to find out what its cation and anion are. Metals are commonly used as positive ions, or cations, in compounds. Anions are the compound’s negative, non-metallic ions. Cations are always produced by metals, while some nonmetals are also suited to creating them.
Stage 4: Identify the polyatomic ions in the process. Polyatomic ions are charged molecules that are so tightly bound together that they remain united during chemical reactions. Polyatomic ions should be recognized because they are charged differently and do not break down into their constituent parts. Polyatomic ions can have both positive and negative charges.
Stage 5: Stabilise the whole molecular equation. Before developing a net ionic equation, make sure your initial equation is completely balanced. Once each element has an equal amount of atoms on either side of the equation, coefficients are placed in front of compounds to balance the equation.
Stage 6: Calculate the constituent compounds’ states of matter. In general, you might locate terms in an issue that show the compound’s status of each molecule. A few rules can be used to determine the state of an element or combination.
Stage 7: Determine which species are going to disintegrate in the solution, dividing them into cations and anions. When a species or combination breaks down, it divides into its positive (cation) and negative (anion) components. These are the components that will eventually be balanced by the net ionic equation.
Stage 8: Determine the charge of each dissociated ion. Remember that nonmetals are the negative anion and metals are the positive cation. The group number of an element in the periodic table can be used to determine what component will have which charge. Each ion’s charge in the molecule must likewise be balanced.
Stage 9: Modify the equation considering the soluble ionic compounds’ individual ions. Strong acids and other dissociable or ionizing chemicals will break into two distinct ions. Despite the fact that the equation must stay balanced, the state of matter remains (aq).
Stage 10: The spectator ions are eliminated by neutralizing identical ions on the two sides of the equation. We can only cancel it if they are exactly the same on both sides. Remove all canceled components from the action.
Net Ionic Equation Examples with Answers
Net Ionic Equation Examples with answers are given here, try to solve them without the help of the solution first.
Example 1: Calculate the net Ionic equation of the specific chemical equation given here.
Mg(NO3)2 + Na2CO3 → MgCO3 + 2 NaNO3
Solution:
The Ionic net equation is –
Mg2+ + 2 NO3¯ + 2 Na++ CO32- → MgCO3 + 2 Na+ + 2 NO3¯
Concise net ionic equation: Mg2+ + CO32- → MgCO3
Example 2: Write the net ionic equation that reflects the reaction of aqueous sodium chromate solution and lead(ll) nitrate, which reacts to generate a yellow precipitate of lead(ll) chromate and aqueous sodium nitrate.
Solution:
The ionic equation is as follows –
2Na+(aq) + CrO42-(aq) + Pb2+(aq) + 2NO3–(aq) –> PbCrO4(s) + 2Na+(aq) + 2NO3–(aq)
The Ionic net equation is –
CrO42-(aq) + Pb2+(aq) –> PbCrO4(s)
Example 3: Compute the net Ionic equation of the given below chemical equation.
MnCl2 + (NH4)2CO3 → MnCO3 + 2 NH4Cl
Solution:
The Ionic net equation is –
Mn2+ + 2 Cl¯ + 2 NH++ CO32- → MnCO3 + 2 NH4+ + 2 Cl¯
The shortened net ionic equation is Mn2+ + CO32- → MnCO3


 NEET UG 2025: Is NEET Previous years Que...
NEET UG 2025: Is NEET Previous years Que...
 JEE Mains Session 2 Result 2025 OUT, Sco...
JEE Mains Session 2 Result 2025 OUT, Sco...
 TS Inter Results 2025 Date for TSBIE 1st...
TS Inter Results 2025 Date for TSBIE 1st...










