Table of Contents
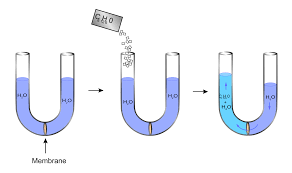
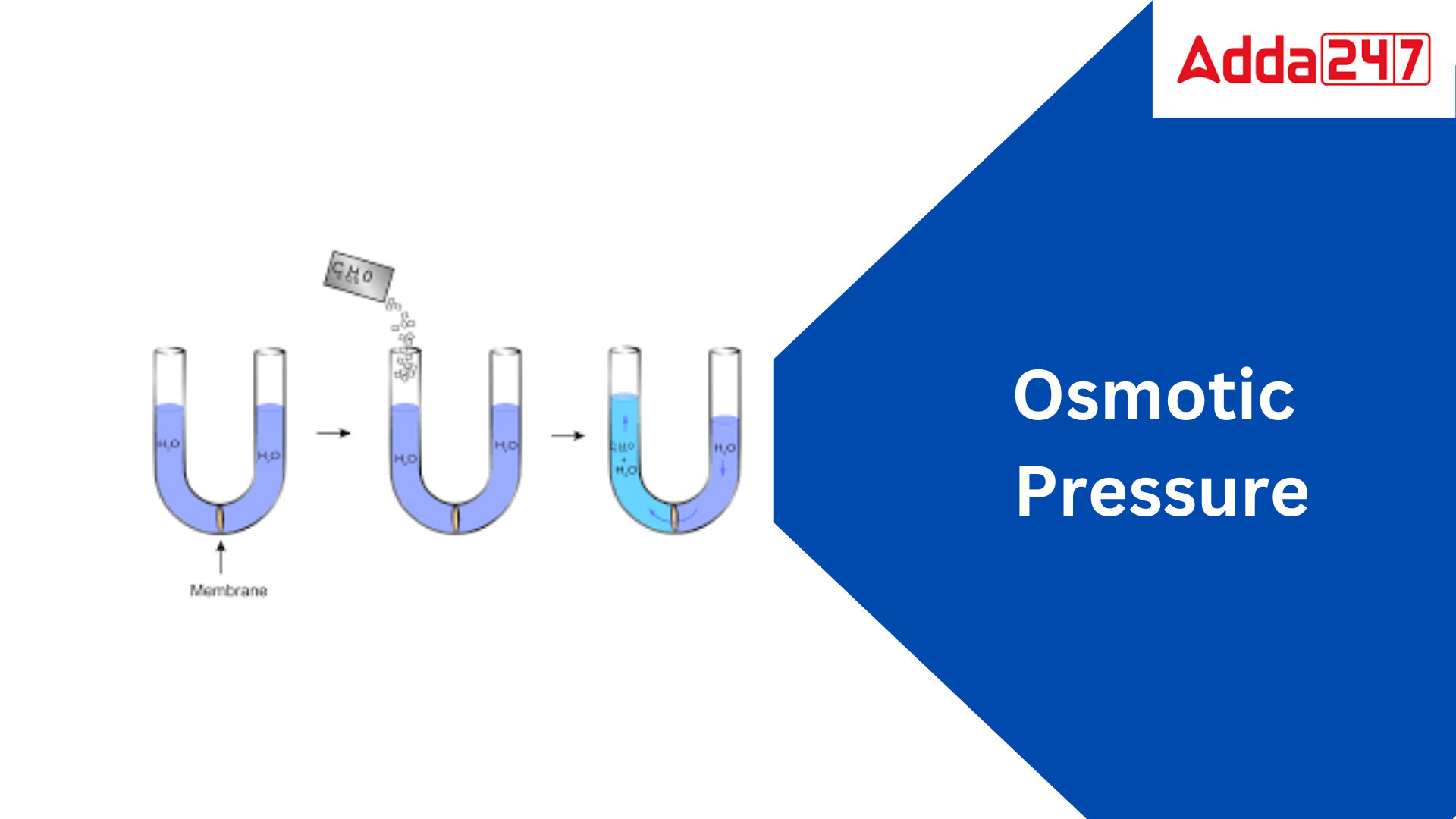
Osmotic Pressure: Osmotic pressure is the pressure applied by a solution to prevent the inward flow of water through a semipermeable membrane. The membrane permits water molecules to pass through but not solute molecules. When two solutions of differing concentrations are separated by a semipermeable membrane, the solution with the higher concentration of solute exerts a stronger osmotic pressure. This is because the water molecules will transfer from the solution with the lower concentration of solute to the solution with the higher concentration of solute in an attempt to equalise the concentrations.
Osmotic pressure is a significant phenomenon in many biological and chemical processes. It is, for example, in charge of plant cell turgor pressure, which helps to keep the cells rigid and upright. Osmotic pressure is also employed in a range of industrial operations, such as seawater desalination and water purification.
What is Osmotic pressure?
Osmotic pressure is defined as the minimal pressure required to stop the flow of solvent molecules across a semipermeable membrane (osmosis). It is a colligative property that is affected by the solute particle concentration in the solution. The following formula can be used to compute osmotic pressure:
π = iCRT
Where,
The osmotic pressure is
The van’t Hoff factor is i.
R is the universal gas constant, and C is the molar concentration of the solute in the solution.
The letter T stands for temperature.
The Dutch chemist Jacobus van’t Hoff proposed this link between a solution’s osmotic pressure and the molar concentration of its solute. It should be noted that this equation only applies to solutions that act like perfect solutions.
Osmotic pressure is the pressure applied by a solution to prevent water from passing through a semipermeable barrier. The membrane permits water molecules to pass through while preventing solute molecules from passing through. When two solutions of differing concentrations are separated by a semipermeable membrane, the solution with the higher solute concentration exerts greater osmotic pressure. This is because, in an attempt to equalise the concentrations, the water molecules will transfer from the solution with the lower concentration of solute to the solution with the higher concentration of solute.
Osmotic Pressure Formula
Osmotic pressure is a significant phenomenon in a wide range of biological and chemical processes. For example, it is in charge of plant cell turgor pressure, which helps to keep the cells rigid and upright. Osmotic pressure is also used in a variety of industrial operations, including seawater desalination and water purification.
Here is the osmotic pressure formula:
π = iCRT
where:
The osmotic pressure is
The van’t Hoff factor is i.
R is the gas constant and C is the solute’s molar concentration.
The absolute temperature is denoted by T.
The van’t Hoff factor is a dimensionless variable used to calculate the number of particles generated when a solute dissolves in water. The van’t Hoff factor for NaCl, for example, is 2 because NaCl dissolves to generate Na+ and Cl- ions, which are two particles.
The gas constant is a physical constant worth 0.08206 Latmmol-1K-1.
The temperature in Kelvin is the absolute temperature.
A variety of methods, including the osmometer, can be used to measure osmotic pressure. Osmometers are instruments that measure the difference in pressure between two liquids separated by a semipermeable membrane.
The notion of osmotic pressure is significant in many domains, including biology, chemistry, and engineering. It is a fundamental property of solutions with numerous applications.
What happens when a force greater than the osmotic pressure is applied to the solution side?
The solvent molecules will flow from the solution to the solvent side when a force greater than the osmotic pressure is applied to the solution side. This is due to the force overpowering the solvent molecules’ tendency to shift from the side with a lower solute concentration to the side with a greater solute concentration.
This is known as reverse osmosis. Reverse osmosis is utilised in a range of applications, including seawater desalination and water purification.
Here’s an example of reverse osmosis in action:
- Desalination: Seawater is a salt-water solution. The salt concentration in saltwater is substantially higher than that of pure water. This means that the osmotic pressure of seawater is greater than that of pure water.
- Water purification: Reverse osmosis can be used to purify water that contains contaminants such as dissolved salts or minerals. Because the contaminants are dissolved in the water, they have a lower osmotic pressure than pure water.
- When a force larger than the osmotic pressure is applied to the solution side, the solvent molecules flow from the solution to the solvent side, bringing contaminants with them. This method will finally eliminate all contaminants from the water, leaving clean water left.
Applications of Osmotic Pressure
Osmotic pressure is a very important concept in many fields, including biology, chemistry, and engineering. It has a wide range of applications, some of which are listed below:
- Desalination: Osmotic pressure can be used to desalinate seawater, which is the process of removing salt from seawater. This is done by applying a pressure greater than the osmotic pressure of seawater to the seawater side of a semipermeable membrane. This forces the water molecules from the seawater to the pure water side of the membrane, leaving behind the salt.
- Purification of water: Osmotic pressure can also be used to purify water. This is done by passing the water through a semipermeable membrane that allows water molecules to pass through but not other impurities. The impurities are then removed from the water, leaving behind pure water.
- Food preservation: Osmotic pressure can be used to preserve food. This is done by placing the food in a solution that has a higher osmotic pressure than the food. This causes the water molecules in the food to move out of the food and into the solution, which helps to prevent the growth of bacteria and other microorganisms.
- Dialysis: Osmotic pressure is used in dialysis, which is a process that is used to remove waste products from the blood. In dialysis, the blood is passed through a semipermeable membrane that allows water and small molecules to pass through but not larger molecules such as proteins. The waste products are then removed from the blood and replaced with fresh water.
- Reverse osmosis: Reverse osmosis is a process that is used to produce pure water from seawater or other salty water. In reverse osmosis, a pressure greater than the osmotic pressure of the water is applied to the water, which forces the water molecules to move from the salty water to the pure water side of a semipermeable membrane.
Osmotic Pressure in the Body
Osmotic pressure is a key element in fluid balance in the body. Extracellular fluid (ECF) surrounds the cells in the body. The ECF contains dissolved salts and other substances. The concentration of these solutes varies between within and outside the cells. This difference in concentration generates osmotic pressure, which aids in keeping cells from swelling or contracting.
This difference in concentration produces osmotic pressure, which aids in the prevention of cell swelling or shrinkage.
The dissolved solute concentration determines the ECF’s osmotic pressure. The greater the concentration of solutes in the ECF, the greater the osmotic pressure. The concentration of solutes within the cells also influences the osmotic pressure of the cells. The higher the osmotic pressure, the more solutes inside the cells.
The cells are said to be in balance when the osmotic pressure of the ECF equals the osmotic pressure of the cells. This means that no water flows into or out of the cells. If the ECF’s osmotic pressure differs from the cells’, water will migrate across the cell membrane to equalize the concentrations.
If the ECF’s osmotic pressure is greater than the cells’ osmotic pressure, water will flow out of the cells, causing them to shrink. Hypotonic shock is the medical term for this. If the ECF’s osmotic pressure is lower than the cells’, water will migrate into the cells, causing them to swell. Hypertonic shock is the medical term for this. The ability of the body to maintain homeostasis is greatly influenced by osmotic pressure. It prevents cells from swelling or shrinking, which can cause damage and major health concerns.




 NEET City Intimation Slip 2025 Available...
NEET City Intimation Slip 2025 Available...
 When is ICSE 10th Result 2025? ReleaseTi...
When is ICSE 10th Result 2025? ReleaseTi...
 Most Repeated Previous year Questions in...
Most Repeated Previous year Questions in...






