Oxalic Acid Formula: Oxalic acid is an organic chemical found in a variety of vegetables and plants. In Organic Chemistry the Oxalic acid formula is C2H2O4. Oxalic acid is the most basic dicarboxylic acid, with only two carboxylic acid groups (COOH) that are directly connected to each other at the carbon atoms. The oxidation of carbohydrates results in the formation of oxalic acid. The most significant application of it is in the cleaning business, such as laundries, bleaching, dyeing, and so on. In this article, we are going to learn about the Oxalic Acid Formula, its preparation, and its Applications in this article
What is Oxalic Acid?
Oxalic acid is the most basic dicarboxylic acid, with only two carboxylic acid groups directly linked to the carbon atom. Ethanedioic Acid is the IUPAC designation for oxalic acid. Oxalic acid appears as a white crystalline solid that dissolves in water to generate a colorless solution. Oxalic acid is present in a variety of vegetables and plants. Oxalic acid is a toxic crystalline acid found in plants such as rhubarb leaves, wood sorrel, and others.
Oxalic Acid Formula
Oxalic acid has the chemical formula C2H2O4.. Oxalic acid is a powerful dicarboxylic acid that is much more acidic than acetic acid, which comprises Carbon(C), Hydrogen(H), and Oxygen(O) in a 1:1:2 ratio. But Oxalic acid acts weak acid that will only partially ionize in water. Oxalic acid has two protons that are acidic. The initial ionization produces HC2O4-, a weak acid that will also ionize. Oxalic acid has just two carboxylic acids directly connected at carbon atoms, i.e. (COOH) groups. As a result, oxalic acid has the formula HOOC-COOH, which can also be written as (COOH)2 or C2H2O4.
Oxalic Acid Structure
As the Oxalic Acid formula says, it has only two carboxylic acids that are physically bonded at carbon atoms, Oxalic acid exists in two distinct polymorphs in its anhydrous form. Hydrogen bonding happens at the intermolecular level and results in a chain-like structure. This combonding’s second polymorph. Check out the Oxalic Acid Structure below.
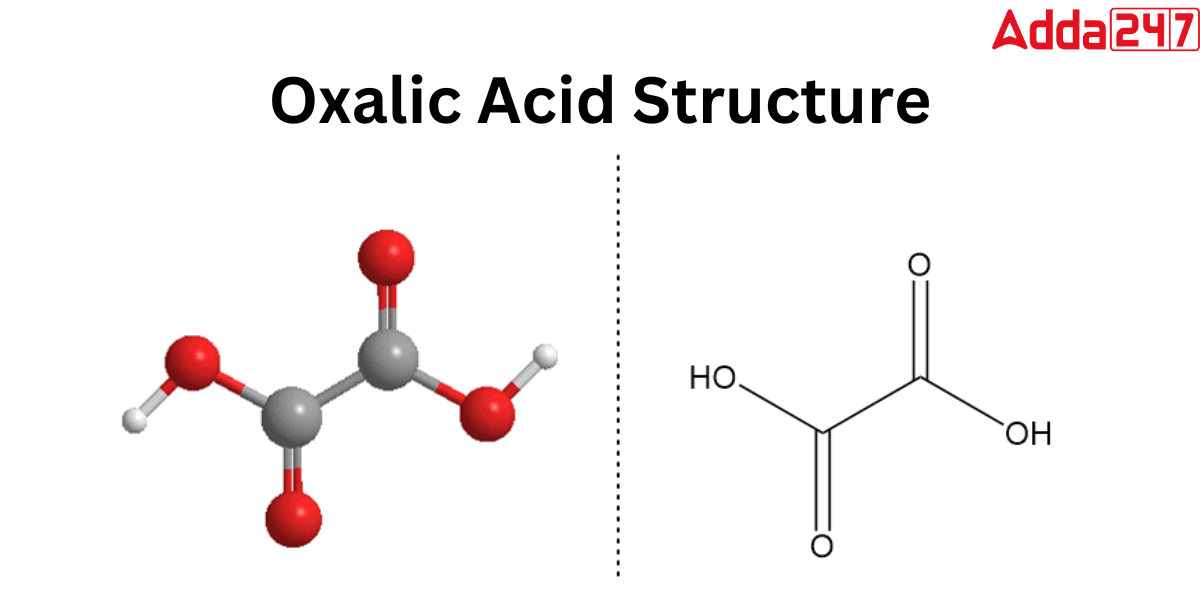
Oxalic Acid Molecular Weight
Oxalic Acid: C2H2O4. There are 2 atoms of Carbon, 2 atoms of Hydrogen and 4 atoms of Oxygen. Therefore, molar mass of oxalic acid (C2H2O4) = 90g/mol.
Oxalic Acid Equivalent Weight
Earlier we came to know that, The Chemical formula for Oxalic Acid is represented as COOH-COOH. As a result, it is obvious that it can give two H+ ions. Hydrated oxalic acid has a molar mass of 126 grams per mole, So, using this method, we can compute the equivalent weight of oxalic acid:
Oxalic Acid Equivalent weight = (molecular weight)/(number of equivalent moles)
The equivalent weight of oxalic acid is as follows:
Oxalic acid equivalent mass = oxalic acid molecular mass/2 = 126g/2 = 63 grams.
As a result, the oxalic acid equivalent weight is 63 grams.
Oxalic Acid Properties
The Properties of Oxalic Acid are listed below:
- It is the most basic dicarboxylic acid, with a higher acidic strength than acetic acids.
- It is easily soluble in water, where it creates a colorless, acidic solution.
- Oxalic acid is a colorless, crystalline white crystal with no odor.
- It has a density of 1.90 g/cm3.
- It has a melting point of 190 °C.
- Anhydrous oxalic acid is highly hydrophilic and can absorb water.
- The molar mass of oxalic acid is 90 g/mol, whereas the molar mass of hydrated oxalic acid is 126 g/mol.
- The valence coefficient of oxalic acid is 2.
Preparation of Oxalic Acid
Oxalic acid and its many oxalate derivatives are found in an assortment of natural sources and can be recovered using various methods. However, oxalic acid may also be produced artificially for laboratory and commercial usage.
Laboratory Preparation of Oxalic Acid
In the laboratory, the most typical way of producing oxalic acid is to oxidize specific carbohydrates such as sucrose with a strong oxidizing agent such as nitric acid in the presence of a catalyst such as vanadium pentoxide. The CHOH units in sucrose combine to generate the di-carboxylic group of oxalic acid. The subsequent reaction is,
C12H22O11 + 18[O] (HNO3 and V2O5) —-> (COOH)2 + H2O
Commercial Preparation of Oxalic Acid
One of the most common applications for oxalic acid is in the cleaning sector, such as laundries, bleaching, dyeing, and so on. We commercially manufacture oxalic acid by processing carbohydrates with nitric acid in the presence of a catalyst vanadium. Glycolic acid or even ethylene glycol can be the carbohydrate precursor that undergoes hydrolysis to create the chemical of interest.
Oxalic Acid Applications
The Various Applications of Oxalic acid are as follows:
- Oxalic acid can be used to eliminate rust and is also useful for bleaching and cleaning.
- It is used as a mordant in the dyeing process.
- It can be used to remove calcium from effluent.
- Beekeepers can utilize oxalic acid in vaporized form.
- Oxalic acid can be used to remove stains from a variety of materials, including ink, food, and mustard.
- It is used to make marble sculptures gleam.
- It is used in the production of dye.
- It is employed as a reducing agent in the development of photographic films.
Health Hazard Due to Oxalic Acid
Oxalic acid is a highly toxic substance. Although oxalic acid is created in the body through the metabolism of ascorbic or glyoxylic acid. Excessive oxalic acid ingestion might be hazardous. Vomiting, diarrhea, severe gastrointestinal disease, kidney damage, shock, convulsions, and coma are all hazardous signs of consumption. Cardiovascular collapse can lead to death. Inhalation can severely harm mucosal membranes and the respiratory tract. Oxalic acid is an eye, mucous membrane, and skin irritant. Kidney injury may result from inhalation or ingestion.









 CBSE Admit Card 2026 for Private & R...
CBSE Admit Card 2026 for Private & R...
 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...














