Oxidation: In science class 9 or 10, almost everyone gets introduced to terms like oxidation and reduction. In simple terms, oxidation is the addition of an oxygen atom to a molecule or the loss of an electron. The inverse of this process is called reduction, and it involves an atom or ion gaining one or more electrons. In any form of reaction, two major types of chemical changes occur: oxidation and reduction. In this post, we will look at what the oxidation process is, what causes it, how oxidation and reduction occur at the same time, examples, and more.
Oxidation Definition
What is the definition of oxidation? The oxidation process can be explained in numerous ways. Oxidation is the removal of electrons within a molecule, atom, or ion reaction, according to the electron concept. When an atom, molecule, or even an ion comes into touch with oxygen, it oxidizes. When this occurs, it changes to achieve a greater degree of stability in its electron shells and transfers electrons as a result. When the oxidation state of a molecule, atom, or ion increases, this is referred to as oxidation. The inverse process is called reduction, and it occurs when electrons are gained when the oxidation status of an atom, molecule, or ion lowers.
What is oxidation in chemistry?
In the Classical definition of oxidation in chemistry, oxygen is introduced to a molecule whenever oxidation occures. While the addition of oxygen to a molecule usually results in electron loss and an increase in oxidation state. This definition of adding oxygen was offered since oxygen gas (O2) was the first recognised oxidising agent. Other sorts of chemical reactions were added to the notion of oxidation. Let’s learn about oxidation in chemistry by looking at the reaction below:
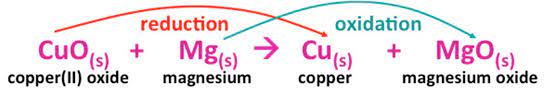
The Mg ion loses electrons in the preceding process to generate magnesium oxide (Oxidation Reaction). CuO, on the other hand, gains an electron to become pure Cu /copper (Reduction Reaction).
Oxidation Examples
The reaction between hydrogen and fluorine gas to generate hydrofluoric acid is an example of a oxidation and reduction reaction. the balanced equation is given below.
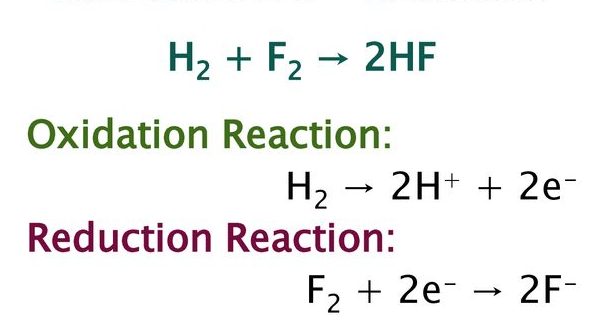
Hydrogen is oxidised and fluorine is reduced in this process. If the reaction is expressed in terms of two half-reactions, it may be easier to understand. There is no oxygen present in this reaction.
H2 → 2 H+ + 2 e-
F2 + 2 e- → 2 F-
Oxidation Reactions
When several elements and the structure of atoms were unknown, the word oxidation was developed. Even while the term is still used today, it now has a broader meaning. Oxidation occurs not only with elements that combine with oxygen, but also with other non-metallic elements. The electron configuration of atoms alters during such events, providing stability. Because oxygen is a reactive element, this stable electron number in atoms can be obtained through a reaction with oxygen. Oxidation occurs when elements or compounds come into contact with oxygen and react. Some examples of oxidation reactions include adding oxygen molecules, eliminating hydrogen, and adding electropositive elements.
- Adding oxygen:
C + O2 → CO2 (oxidation of carbon)
- Elimination of hydrogen:H2S + Br2 → 2 HBr + S (oxidation of sulphide)
- The addition of an electronegative element:
Fe + S → FeS (oxidation of Iron)
- Elimination of electropositive elements:2 KI + H2O2 → I2 + 2 KOH (oxidation of iodide)
Oxidation Process
An oxidation-reduction process, also known as a redox reaction, is one in which electrons are transferred between chemical species (the atoms/ ions involved in the reaction). Redox reactions are all around us: the combustion of fuels, metal corrosion, and even photosynthesis and cellular respiration include oxidation and reduction. The following is a examples of common redox reactions.
Oxidation Involving Oxygen
As previously discussed, oxygen has been supplied to a chemical as an older technique of oxidation. Although oxygen is normally given to a substance to meet the criteria for electron loss and an increase in oxidation state, the oxidation idea has been expanded to include additional chemical processes.
Example – A typical example of an oxidation reaction is the old oxidation notion of iron reacting with oxygen to form iron oxide. Rust formed as the iron oxidised. The oxidation of iron oxide results in the formation of rust. The following is the chemical reaction:
2Fe + O2 → Fe2O3
Oxidation Involving Hydrogen
The contemporary definition of oxidation is oxygen-based oxidation. There is another way to describe hydrogen that can be employed in organic chemistry. This is the inverse of the oxygen notion. According to the description, oxidation results in hydrogen loss, whereas reduction results in hydrogen gain.
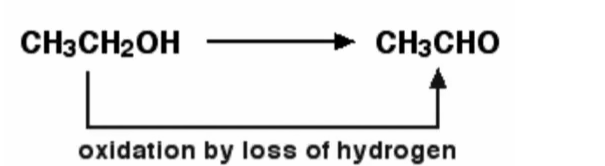
Example – When ethanol is generated by oxidising ethanol, this is an example of this. The loss of hydrogen is known to oxidise ethanol. Ethanol can be decreased by inverting the equation and supplying hydrogen to it. The chemical reaction is demonstrated below.
OILRIG in Chemistry
- OILRIG stand for – Oxidation Is Loss, Reduction Is Gain. This is a useful abbreviation for the terms electron loss or gain. If an atom receives or loses an electron, it becomes an ion.
- This mechanism is defined by whether an atom has lost (oxidation) or gained (reduction) an electron.
- This is an abbreviation for the term used to denote the loss or gain of electrons. It is a straightforward strategy for determining and remembering which elements are oxidised and which are reduced.
Example – if chlorine (Cl) changes to Cl- (a negatively charged ion), we know it has received an electron since electrons have a negative charge. As a result, we can claim that the chlorine atom has been reduced and that a reduction process has happened.
Oxidation and Reduction
- A redox reaction, which stands for reduction-oxidation, is a type of chemical reaction that involves both oxidation and reduction.
- Scientists realised oxidation and reduction occur together after discovering the electron and explaining chemical reactions with electron theory. One species loses electrons and is known as an oxidised element, while another gains electrons and is known as a reduced element.
Redox Reaction Example – metal oxidation by oxygen gas may be interpreted as the metal atom losing electrons to make the cation (being oxidised) and the oxygen molecule receiving electrons to form oxygen anions. For magnesium, for example, the reaction may be reformulated as follows:
2 Mg + O2 → 2 [Mg2+][O2-]
Magnesium ion and oxide ions are shown below.
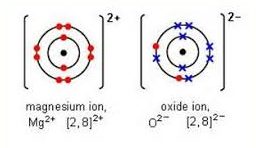
made up of the half-reactions listed below:
Mg → Mg2+ + 2 e-
O2 + 4 e- → 2 O2-
Oxidizing agents
Important terminology used to describe the reactants in redox reactions that move electrons between reactants to generate products include oxidising and reducing agents.
Oxidising agent:
In a chemical process, an oxidising agent, also known as an oxidant, obtains electrons and is reduced. The oxidising agent, also known as the electron acceptor, is generally in one of its higher potential oxidation states since it will receive electrons and be reduced.
Halogens, potassium nitrate, and nitric acid are examples of oxidising agents.
Reducing agent
In a chemical process, a reducing agent, also known as a reductant, loses electrons and is oxidised. A reducing agent is known as an electron donor when it is in one of its lower possible oxidation states. Because it loses electrons in the redox reaction, a reducing agent is oxidised.
Examples earth metals, formic acid, and sulfite compounds.








 Haloalkanes and Haloarenes NEET Notes, C...
Haloalkanes and Haloarenes NEET Notes, C...
 d -and f -Block Elements NEET Notes, Che...
d -and f -Block Elements NEET Notes, Che...
 CBSE Class 11 Psychology Syllabus 2025-2...
CBSE Class 11 Psychology Syllabus 2025-2...












