Table of Contents
The Punjab School Education Board (PSEB) has released the PSEB Class 12 Chemistry Model Test Paper 2025 for students in class 12 Science Streams. Along with Chemistry, The board has issued the Punjab board Class 12 Model Questions papers for all students on the website at pseb.ac.in.The importance of solving sample papers cannot be overstated, it provides an excellent opportunity to assess your level of comprehension and knowledge. On this page, we present the Complete PSEB Class 12 Chemistry Model Test Paper 2025 along with the official downloadable PDF to make things easier.
PSEB Class 12 Chemistry Model Test Paper 2025
PSEB Class 12 Chemistry Model Test Paper 2025 is an important element of exam preparation that allows students to assess the exam pattern as well as the type of questions that will be asked in the upcoming board exam. If you are a class 12 student from the Punjab School Education Board (PSEB) from the science stream and looking for Chemistry model question papers, you have come to the right spot. Here have shared the most recent PSEB 12th Chemistry Model Papers, provided by the board.
Punjab Board 12th Model Question Paper Pattern
Practicing the Class 12 Chemistry sample question paper is one of the most effective ways to improve your grades on the board exams. This helps pupils grasp the exam’s difficulty level and the structure of the question papers. Sample papers allow students to assess how much they have understood from what they have learned.
Taking the schematic distribution of marks of the PSEB 12th Sample question paper, It is worth 70 marks and consists of 21 questions. The questions were divided as follows:
- Question No. 1 had 20 parts carrying 1 mark each (MCQs & True/False)
- Question No. 2 to 15 were for 2 marks each.
- Question No. 16 to 19 were for 3 marks each.
- Question No. 20 and 21 were for 5 marks each
- All questions were compulsory, however, internal choices were provided in some questions.
Punjab Board Class 12 Chemistry Model Test Paper 2025
(MCQs & True/False) Question 1 contains 20 parts of 1 mark each.
Q1. Choose the correct answer
(i) An unripe mango placed in a concentrated salt solution to prepare pickle, shrivels becausea.
a. It gains water due to osmosis.
b. It loses water due to osmosis.
c. It gains water due to reverse osmosis.
d. It loses water due to reverse osmosis.
(ii) In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is?
a. The same b. About twice c. About three times d. About six times
(iii) a-Isotonic solution have
a. same boiling point
b. same vapour Pressure
c. Same melting point
d. Same osmotic Pressure
(iv) What is the final oxidation state of manganese after the electrochemical reactions in a Dry Cell
a. +4 b. +3 c. +2 d. +1
(v) If the unit of specific rate constant (k) for a certain gaseous reaction is atm-2s-1, then, the order of the reaction is
a. Zero order b. First order
c. Second order d. Third order
(vi) The coordination number of platinum in [PtCl2(C5H5N)(NH3)] is
a. 3 b. 4
c. 5 d. 6
(vii) The reaction of toluene with Cl2 in the presence of FeCl3 gives predominantly
a. benzoyl chloride b. benzyl chloride
c. m-chlorotoluene d. o- and p- chlorotoluene
(viii) Which of the following is most reactive towards nucleophilic addition reactions?
a. CH3COCH3 b. CH3CHO
c. CH3COC2H5 d. HCHO
(ix) Which of the following reagents cannot be used to distinguish between pentanal and 2-pentanone?
a. Tollen’s reagent b. Fehling’s solution
c. Br2 in CCl4 d. I2 in NaOH
(x) Which of these is most acidic?
a. CF3COOH b. CCl3COOH
c. CBr3COOH d. CH3COOH
True/False
(xi) The compounds [Co Cl2 (NH3)4] NO2 and [Co Cl (NO2) (NH3)4] Cl show coordination isomerism.
(xii) The crystal field splitting Δo, depends on the field produced by the ligand and charge on the metal ion.
(xiii) The boiling point of ethers are higher than those of isomeric alcohols.
(xiv) Benzaldehyde cannot undergo Cannizzaro reaction.
(xv) The red brown precipitate of Aldehydes with Fehling’s solution is due to the formation of Cu2O
Read the passage and answer the questions (xvi) to (xx)-
Carbohydrates are optically active polyhydroxy aldehydes and ketones or those compounds which on hydrolysis give such compounds are also carbohydrates. The carbohydrates which are not hydrolysed are called monosaccharides. Monosaccharides with aldehydic group are called Aldoses and those with free Ketonic group are called Ketoses. Carbohydrates are optically active. Number of optical isomers= 2 n, where n= number of asymmetric carbons. Carbohydrates are mainly synthesised by plants during photosynthesis. The monosaccharides exist in the form of cyclic structures. In cyclization, the -OH group combines with the aldehydic or ketonic group. As a result, cyclic structures of five or six membered rings containing one oxygen are formed e.g. Glucose, Fructose, Galactose.
(xvi) What are carbohydrates?
(xvii) What are Aldoses?
(xviii) Define Monosaccharides.
(xix) Name a monosaccharide.
(xx) Glucose molecule has four asymmetric carbons. Find the total number of optical isomers in glucose.
Questions 2 to 15 are of 2 marks each.
Q2. The boiling point of a solution containing 1.5g of dichlorobenzene in 100g of benzene was higher by 0.268 K. Calculate the molar mass ofdichlorobenzene. (Kb for benzene is 2.62 degree/molal)
ORCalculate the number of molecules of Oxallic acid (H2C2O4.2H2O)in 100 mL of 0.2 N oxalic acid solution.
Q3. Shazia removed the outer hard shells of two different eggs. She then placed one egg in pure water and the other egg in a saturated solution of sucrose. What change is she likely to observe in the eggs after few hours?
Explain it. (1+1)
Q4. The conductivity of a 0.00241 M acetic acid is 7.896 x 10-5 S cm-1. Calculate its molar conductivity. If Λo for acetic acid is 390.5 S cm²mol-1, what is its degree of dissociation (α)? (1+1)
Q5. Write down the functions of a salt bridge in an electrochemical cell.
Q6. The rate constant of a reaction at 500 K and 700 K are 0.02 s-1 and 0.07 s-1 respectively. Calculate the value of Ea (Activation energy).
OR
Consider the reaction:
4 NO2 (g) + O2 (g) —> 2 N2O5 (g)
In an experiment, the rate of disappearance of O2 is 0.24 mol L-1 s-1. Calculate (I) the rate of disappearance of NO2 and (ii) the rate of formation of N2O5. (1+1)
Q7. Define: (i) Half life of a reaction (ii) Pseudo first order reaction (1+1)
Q8. Transition metals form alloys with other transition metals. Explain why?
Q9. Write down the IUPAC names of-
(i) Na [PtBrCl(ONO)(NH3)] (ii) [Ag(NH3)2] [Ag(CN)2] (1+1)
Q10. (i) Define coordination number (1)
(ii) What is the hybridisation and structure of [Ni(CN)4]- (1)
Q11. How will you convert phenol to salicylaldehyde?
OR
Explain the mechanism of acidic dehydration of ethyl alcohol to form ethene.
Q12. Write down the following reactions-
(i) Aldol condensation (ii) HVZ reaction (1+1)
OR
Explain why carboxylic acids exist as associated molecules?
Q13. Alkylamines are more basic than ammonia. Explain why?
Q14. Write down the following reactions-
(i) Carbylamine reaction (1)
(ii) Reaction between benzene diazonium chloride and phenol in basic medium (1)
Q15. Differentiate between fibrous and globular proteins.
Questions 16 to 19 are of 3 marks each.
Q16. Three electrolytic cells A, B and C containing electrolytes of zinc sulphate, silver nitrate and copper sulphate respectively were connected in series. A steady current of 1.5 amp was passed through them until 1.45 g of silver were deposited at the cathode of cell B.
(i) How long did the current flow? (1)
(ii) What weight of copper and zinc get deposited? (2)
(Atomic masses of Zinc, Silver and Copper respectively are 65.3 g, 108 g and 63.5 g)
OR
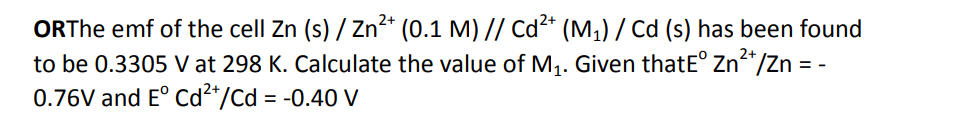
Q17. Starting from 100 g of a radioactive substance, 2.5 g was left after 5 years.
If its radioactive decay follows first order kinetics, calculate-
(i) Rate constant for the decay of the radioactive substance (1)
(ii) The amount of substance left after one year (1)
(iii) The time required for half of the substance to decay. (1)
Q18. Complete the following reactions:
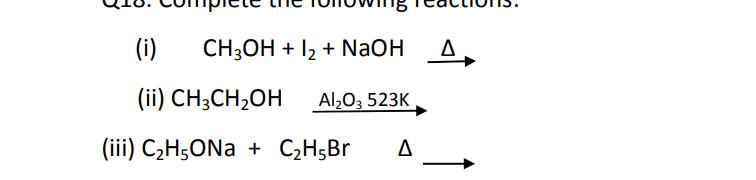
OR
What is Lucas reagent? Write down Lucas test for distinction between primary, secondary and tertiary alcohol. (1+2)
Q19. (i) Lower aliphatic amines are soluble in water. Why? (1)
(ii) Write down a test to distinguish between aromatic primary amines from aliphatic primary amines. (2)
Questions 20 and 21 are of 5 marks each.
Q20. (i) Which Element of 3d transition series has lowest enthalpy of atomisation and why? (1+1)
(ii) Transition elements or their compounds act as catalysts. Explain why. (3)
OR
(i) Define Lanthanoid contraction. (1)
(ii) Why do Ce and Tb show +4 oxidation state? (2)
(iii) Write down two similarities between Lanthanoids and Actinoids. (2)
Q 21. (i) Write down the following reactions:-
a. Sandmeyer reaction
b. Hoffmann ammonolysis reaction
c. Wurtz Fittig reaction
d. Finkelstein Reaction
e. Friedel craft’s Alkylation (5)
OR
(i) Explain the mechanism of Substitution Nucleophilic bimolecular reactions of Haloalkanes with a suitable example. (3)
(ii) Explain giving two reasons why Haloarenes are less reactive towards Nucleophilic substitution reactions than Haloalkanes.
PSEB Class 12 Chemistry Model Test Paper 2025 PDF
If you want to download the PSEB Class 12 Chemistry Model Test Paper 2025 in PDF format then, we are advised to visit the official website pseb.ac.in. However, for the convenience of the students, we have shared the direct link to download the Punjab Board Class 12 Chemistry Model Test Paper 2025 PDF Below.





 CBSE Class 12 Psychology Question Paper ...
CBSE Class 12 Psychology Question Paper ...
 CBSE Sample Paper 2024-25 Class 10 with ...
CBSE Sample Paper 2024-25 Class 10 with ...
 CBSE Class 12 Biology Question Paper 202...
CBSE Class 12 Biology Question Paper 202...










