Table of Contents
Photoelectric Effect Notes: The notes on the Photoelectric Effect will not only help students in their board examinations but will also help in clearing competitive exams like NEET. It is one of the few topics that permeate both physics and chemistry. The concept of the Photoelectric Effect helped establish the particle nature of the light. The great Albert Einstein made huge contributions in this area of study.
Photoelectric Effect
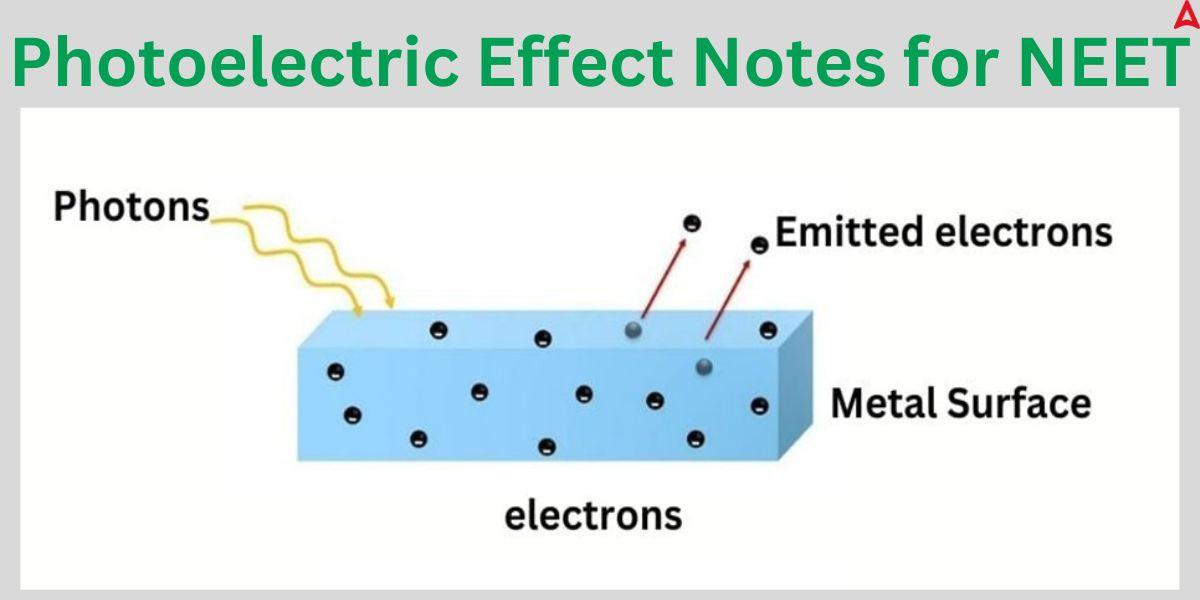
The photoelectric effect happens when electrons on the surface of a metal absorb light and overcome the forces of attraction holding them to metal nuclei or cores. When the forces become strong enough to overcome the threshold force (also known as work function), the electrons leaves the metal surface. It is impossible to understand the photoelectric effect by thinking of light as a wave. Nevertheless, this behavior can be explained by light’s particle nature, which allows light to be seen as a stream of electromagnetic energy particles. So, it also establishes the particle nature of the light. Let us delve into this concept in more detail.
Due to its great significance in Physics, it is one of the favorite topics of the examiner. Mastering this topic will give students an edge in the board as well as the competitive exams. To help students with their preparation, we have provided students with the expert notes PDF on this topic along with a detailed video explanation by the expert NEET faculty of Adda247.
Photoelectric Effect Notes
The photoelectric effect is one of the crucial concept in the modern physics section of the Physics subject. Due to its multitude application in the modern world, its questions finds a frequent place in the exam. The phenomenon of photoelectric effect occurs when light strikes a metal surface and causes electrons to be released. We refer to these released electrons as photoelectrons. It is significant to remember that the frequency of the light impinge on the metal surface affects both the photoelectron emission and the kinetic energy of the expelled photoelectrons. Photoemission is the general term for the process by which photoelectrons are expelled from the metal surface as a result of being exposed to light.
What is Photoelectric Effect?
The release of electrons from a metal upon the impact of a light beam is known as the photoelectric effect. Photoelectrons are electrons that are released as a result of the photoelectric effect. According to Albert Einstein, light and other electromagnetic radiation are surges of discrete energy particles called photons rather than waves travelling through space.
The concept of conservation of energy is the fundamental idea behind the photoelectric effect. When light strikes an exposed object, a process known as photoemission causes the photoelectrons to be released. The material’s surface electrons absorb light energy and use it to break specific forces connecting them to the nucleus, which is how the photoelectric effect happens.
Photoelectric Effect Definition
As per the formal definition, The process of emission of electrons from the metal surface when a light beam strikes it is known as the Photoelectric Effect. The emission is observed only when the energy of the beam of light is equal to or more than the threshold energy. The beam of light consists of many energy particles called photons. When these photons strike an electron with excessive energy, the electron gets ejected from the metal surface. The minimum amount of frequency required by the photon to eject the electron from the metal surface is known as the threshold frequency.
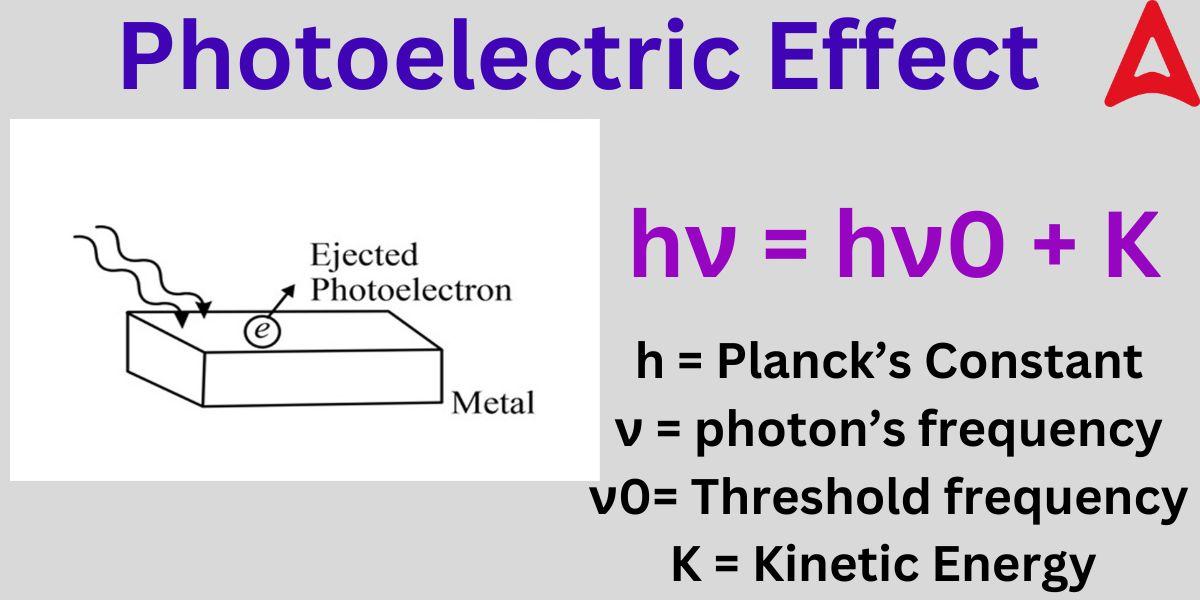
Photoelectric Effect Formula
Like any other physical concept, the concept of the Photoelectric Effect can be quantified.
The Photoelectric Effect can be mathematically expressed by the following formula.
E = W + K
or hν = hν0 + (1/2) x m x v²
where, E = energy of the photon
W = work function (threshold energy)
K = kinetic energy of the electron
ν = frequency of photon
ν0 = threshold frequency
m = mass of the electron
v = velocity of the electron
h = Planck’s constant
Laws of Photoelectric Effect
When observing the photoelectric effect or photoelectric emission, there are specific laws that must be followed. The laws of Photoelectric Effect is stated below.
- There is no or very little time gap between the incident light and the photoelectron emission.
- The photoelectron’s kinetic energy is contingent upon the incident light frequency.
- The quantity of photoelectrons released is directly correlated with the intensity of the incoming light.
- Only frequencies over a certain one, known as the cut-off or threshold frequency, cause photoelectrons to be released.
Photoelectric Effect for NEET
The chapter on the Photoelectric Effect has an equal importance in both Physics and Chemistry NEET syllabus. Every year, round 2-3 questions are asked from this concept. Learning this concept with full heart will help student bag crucial 10-12 marks in the NEET exam. As we know, each mark counts in this highly competitive exam, gaining 10-12 marks from this chapter will strengthen the chance of selection in the NEET exam. Keeping this in mind, in the coming sections, we have provided students with the PDF notes for the Photoelectric Effect concept and a detailed explanation video on the same.
Photoelectric Effect Notes PDF for NEET
All the important notes of the Photoelectric Effect for the NEET exam has been covered in the PDF provided below. The Photoelectric Effect notes PDF will help students learn the concept at their own will even in the absence of the active internet. The PDF have been compiled by the expert NEET faculty for Physics at Adda 247. The PDF contains all the important details of this chapter along with some important questions with solutions. Students can download this important PDF by clicking on the link given below.
Click Here to Download Photoelectric Effect Notes PDF for NEET
Photoelectric Effect Notes Video
Looking at the visuals and understanding the concept firsthand from an expert NEET faculty will not only help students in clearing all the doubts but will also strengthen their concept for this chapter. The video explanation given below presents this whole concept in a simple way. After going through the detailed video and the PDF notes (link given above), students will surely be able to tackle any question on this concept.





 CBSE Class 12 Physics Viva Questions wit...
CBSE Class 12 Physics Viva Questions wit...
 BSc Physics Syllabus 2025: Check Year Wi...
BSc Physics Syllabus 2025: Check Year Wi...
 Physics Investigatory Project Class 12: ...
Physics Investigatory Project Class 12: ...










