Table of Contents
Planck’s Constant
Planck’s constant (denoted by h) is a fundamental physical constant that describes the behavior of particles and waves on the atomic scale, along with the particle component of light, in the mathematical form of quantum mechanics. In his precise definition of the distribution of the radiation emitted by a blackbody, or perfect absorber of radiant energy (see Planck’s radiation law), the German physicist Max Planck proposed the constant in 1900. Radiation, such as light, is emitted, transmitted, and absorbed in discrete energy packets, or quanta, which are determined by the frequency of the radiation and the value of Planck’s constant.
Planck’s Constant Value
Each quantum’s or photon’s energy E is equal to Planck’s constant h multiplied by the radiation frequency indicated by the Greek letter nu, v, or simply E = h. The quantization of angular momentum is done using a modified version of Planck’s constant called h-bar (), or the reduced Planck’s constant, which equals h divided by 2. An electron coupled to an atomic nucleus, for example, has quantized angular momentum that can only be a multiple of h-bar.
The Value of Plank Constant the exact value = 6.62607015×10−34 J⋅Hz−1
Planck’s Constant Definition
The Planck constant, often known as Planck’s constant, is a basic physical constant in quantum mechanics that is symbolised by the letters h h h. The energy of a photon is equal to its frequency times the Plancks constant. The Plancks constant also connects mass and frequency due to mass–energy equivalence. A fundamental natural law is based on the Plancks constant.
Planck’s Constant Formula
The slope of the graph multiplied by e/c, where e is the electronic charge and c is the velocity of light is used to find Planck’s constant.
Also, Planck’s Constant is used in the Planck-Einstein Relation. The energy of light is not transported continually as in a conventional wave, but only in little “packets” or quanta, according to Einstein’s explanation. The size of these “energy packets,” later dubbed photons, was supposed to be the same as Planck’s “energy element,” yielding the contemporary Planck–Einstein relation:
where E is Energy, h is Planck’s constant and f is frequency.
Planck’s Constant Formula in Others words
E = h * ν
In this formula:
- E represents the energy of a photon,
- h represents Planck’s constant, and
- ν represents the frequency of the photon.
Planck’s constant has a value of approximately 6.62607015 × 10^(-34) joule-seconds (J·s) in the International System of Units (SI).
The formula E = h * ν is often used in various areas of physics, such as quantum mechanics and electromagnetism, to calculate the energy of photons or to relate energy to frequency. It is a fundamental equation that forms the basis for understanding the quantized nature of energy in the microscopic world.
Planck’s Constant Symbol
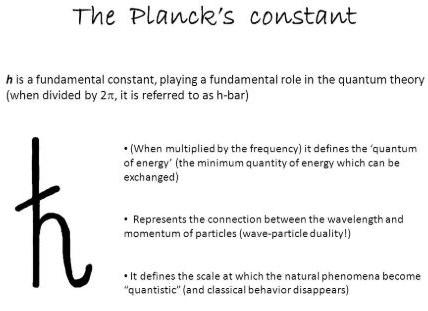
Planck’s Constant Unit is eV and joule
The product of energy multiplied by time, called action, is the dimension of Planck’s constant. As a result, Planck’s constant is frequently referred to as the fundamental quantum of action. It has a value of exactly 6.62607015 × 10−34 joule second in metre-kilogram-second unit and also in the SI units. Its other unit is Electronvolt or eV.
Planck’s Constant- Applications
Following are the applications of the Planck’s constant:
- The Planck length can be calculated using the Planck constant, the speed of light in a vacuum, and the gravitational constant.
- A fundamental natural law is based on the Planck constant. Max Planck established a connection that yielded the Planck constant.
- Another unit obtained indirectly from the Planck constant is the Planck time. Because the Planck time is the amount of time it takes for light to travel, it can be computed immediately once the Planck length is known.
Related Post:



 Anna University Result 2025 OUT at coe1....
Anna University Result 2025 OUT at coe1....
 KCET Physics Question Paper Answer Key 2...
KCET Physics Question Paper Answer Key 2...
 NEET PG 2025 Notification Out at natboar...
NEET PG 2025 Notification Out at natboar...










